Abstract
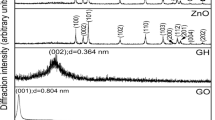
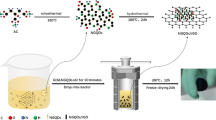
Using oxalic acid as a reducing agent, hydroquinone (HQ), graphene (GO), and acidified carbon nanotubes (CNTs) were compounded directly to synthesize reduced graphene oxide (rGO)/CNTs/HQ hydrogels by a one-step hydrothermal method, and the phase composition and electrochemical properties were studied. During the hydrothermal reaction, the π bond in the hydroquinone molecule will be compounded with the large π bond on the surface of rGO due to the π-π interaction. At the same time, CNTs play a role of spacer and support in rGO/CNTs/HQ and improve the conductivity of rGO/CNTs/HQ. The prepared rGO/CNTs/HQ has a mass-specific capacitance of 219 Fg−1 at 0.5 Ag−1; at 10 Ag−1, the mass-specific capacitance retains 81%, confirming its excellent rate performance; After 2000 cycles, the mass ratio capacitance retains 94%, and the cycle performance is excellent.










Similar content being viewed by others
References
Yang P, Mai W (2014) Flexible solid-state electrochemical supercapacitors[J]. Nano Energy 8:274–290
Zhai Z-B, Huang K-J, Wu X, Hu H, Xu Y, Chai R-M (2019) Metal-organic framework derived small sized metal sulfide nanoparticles anchored on N-doped carbon plates for high-capacity energy storage. Dalton Trans 48(14):4712–4718
Zhai Z-B, Huang K-J, Wu X (2018) Superior mixed Co-Cd selenide nanorods for high performance alkaline battery-supercapacitor hybrid energy storage. Nano Energy 47:89–95
Xing L-L, Huang K-J, Cao S-X, Pang H (2018) Chestnut shell-like Li4Ti5O12 hollow spheres for high-performance aqueous asymmetric supercapacitors. Chem Eng J 332:253–259
Wu X, Zhang H, Zhai Z-B, Xu J, Huang K-J (2020) Tellurium-impregnated P-doped porous carbon nanosheets as both cathode and anode for an ultrastable hybrid aqueous energy storage. J Mater Chem A 8(33):17185–17192
Llx A, Xu WB, Kjh A (2018) High-performance supercapacitor based on three-dimensional flower-shaped Li4Ti5O12-graphene hybrid and pine needles derived honeycomb carbon - ScienceDirect[J]. J Colloid Interface Sci 529:171–179
Wu X, Zhai Z-B, Huang K-J, Ren R-R, Wang F (2020) Boosting energy and power performance of aqueous energy storage by engineering ultra-fine metallic VSe2 nanoparticles anchored reduced graphene oxide. J Power Sources, 448
Sial QA, Javed MS, Lee Y-J, Le TD, Seo H (2020) Flexible and transparent graphene-based supercapacitors decorated with nanohybrid of tungsten oxide nanoflakes and nitrogen-doped-graphene quantum dots[J]. Ceram Int 46(14):23145–23154
Zhang L, Cai P, Wei Z, Liu T, Yu J, Al-Ghamdi AA et al (2021) Synthesis of reduced graphene oxide supported nickel-cobalt-layered double hydroxide nanosheets for supercapacitors[J]. J Colloid Interface Sci 588:637–645
Liu Y, Xin N, Yang Q, Shi W (2021) 3D CNTs/graphene network conductive substrate supported MOFs-derived CoZnNiS nanosheet arrays for ultra-high volumetric/gravimetric energy density hybrid supercapacitor[J]. J Colloid Interface Sci 583:288–298
Gayathri S, Arunkumar P, Han JH (2021) Scanty graphene-driven phase control and heteroatom functionalization of ZIF-67-derived CoP-draped N-doped carbon/graphene as a hybrid electrode for high-performance asymmetric supercapacitor[J]. J Colloid Interface Sci 582:1136–1148
Quoc BL, Haojie F, Bubulinca C, Ngwabebhoh FA, Kazantseva NE, Saha P (2020) Reduced graphene oxide composited with Ni-MOF and PANI applied as electrodes for supercapacitor[J]. ECS Trans 99(1):93–101
Andikaey Z, Ensafi AA, Rezaei B (2020) Synthesis of engineered graphene nanocomposites coated with NiCo metal-organic frameworks as electrodes for high-quality supercapacitor[J]. Int J Hydrogen Energy 45(56):32059–32071
Makkar P, Ghosh NN (2021) High-performance all-solid-state flexible asymmetric supercapacitor device based on a Ag-Ni nanoparticle-decorated reduced graphene oxide nanocomposite as an advanced cathode material[J]. Ind Eng Chem Res 60(4):1666–1674
Xia H, Ragavendran KR, Xie J, Lu L (2012) Ultrafine LiMn2O4/carbon nanotube nanocomposite with excellent rate capability and cycling stability for lithium-ion batteries[J]. J Power Sources 212:28–34
Jia X, Yan C, Chen Z, Wang R, Zhang Q, Guo L et al (2011) Direct growth of flexible LiMn2O4/CNT lithium-ion cathodes[J]. Chem Commun 47(34):9669–9671
Sandhiya M, Balaji SS, Sathish M (2020) Boosting the energy density of flexible supercapacitors by redox-additive hydrogels[J]. Energy Fuels 34(9):11536–11546
Cong H-P, Ren X-C, Wang P, Yu S-H (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process[J]. ACS Nano 6(3):2693–2703
Kabiri S, Tran DNH, Altalhi T, Losic D (2014) Outstanding adsorption performance of graphene-carbon nanotube aerogels for continuous oil removal[J]. Carbon 80:523–533
Kumar GG, Hashmi S, Karthikeyan C, GhavamiNejad A, Vatankhah-Varnoosfaderani M, Stadler FJ (2014) Graphene oxide/carbon nanotube composite hydrogels-versatile materials for microbial fuel cell applications[J]. Macromol Rapid Commun 35(21):1861–1865
Hashmi S, GhavamiNejad A, Obiweluozor FO, Vatankhah-Varnoosfaderani M, Stadler FJ (2012) Supramolecular interaction controlled diffusion mechanism and improved mechanical behavior of hybrid hydrogel systems of Zwitterions and CNT[J]. Macromolecules 45(24):9804–9815
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials[J]. Small 6(6):711–723
Chen, Hamon, Hu, Chen, Rao, Eklund et al (1998) Solution properties of single-walled carbon nanotubes[J]. Science (New York, N.Y.), 282 (5386): 95–98
O’Connell MJ, Boul P, Ericson LM, Huffman C, Yuhuang W, Haroz E et al (2001) Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping[J]. Chem Phys Lett 342(3–4):265–271
Tung VC, Chen L-M, Allen MJ, Wassei JK, Nelson K, Kaner RB et al (2009) Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors[J]. Nano Lett 9(5):1949–1955
Zhang M, Gao B, Cao X, Yang L (2013) Synthesis of a multifunctional graphene-carbon nanotube aerogel and its strong adsorption of lead from aqueous solution[J]. RSC Adv 3(43):21099–21105
Dong X, Chen J, Ma Y, Wang J, Chan-Park MB, Liu X et al (2012) Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water[J]. Chem Commun 48(86):10660–10662
Qiu Z, He D, Wang Y, Li J (2017) Low-temperature fabrication of 3D drilled graphene sheets hydrogel for supercapacitors with ultralong cycle life[J]. Chem Phys Lett 684:290–297
Gao X-L, Wang D-D, Li S, Xing W, Yan Z-F (2018) Hydroquinone-modified mesoporous carbon nanospheres with excellent capacitive performance[J]. J Inorg Mater 33(1):48–52
Ren L, Zhang G, Lei J, Wang Y, Hu D (2018) Novel layered polyaniline-poly(hydroquinone)/graphene film as supercapacitor electrode with enhanced rate performance and cycling stability[J]. J Colloid Interface Sci 512:300–307
Sun Q, He T, Li Y (2020) A novel all solid-state asymmetric supercapacitor with superior electrochemical performance in a wide temperature range using a hydroquinone modified graphene xerogel as the cathode and N-doped Ti3C2Tx as the anode[J]. J Mater Chem A 8(4):1687–1696
Han C, Ye Y, Wang G, Hong W, Feng C (2018) Selective electro-oxidation of phenol to benzoquinone/hydroquinone on polyaniline enhances capacitance and cycling stability of polyaniline electrodes[J]. Chem Eng J 347:648–659
Vonlanthen D, Lazarev P, See KA, Wudl F, Heeger AJ (2014) A STab polyaniline-benzoquinone-hydroquinone supercapacitor[J]. Adv Mater 26(30):5095–5100
Singh C, Paul A (2015) Physisorbed hydroquinone on activated charcoal as a supercapacitor: an application of proton-coupled electron transfer[J]. J Phys Chem C 119(21):11382–11390
Park J, Kumar V, Wang X, Lee PS, Kim W (2017) Investigation of charge transfer kinetics at carbon/hydroquinone interfaces for redox-active-electrolyte supercapacitors[J]. ACS Appl Mater Interfaces 9(39):33728–33734
Zhang ZJ, Chen XY (2018) Illustrating the effect of electron withdrawing and electron donating groups adherent to p-hydroquinone on supercapacitor performance: the cases of sulfonic acid and methoxyl groups[J]. Electrochim Acta 282:563–574
Barua A, Paul A (2020) Unravelling the role of temperature in a redox supercapacitor composed of multifarious nanoporous carbon@hydroquinone[J]. RSC Adv 10(3):1799–1810
Xie H, Zhu Y, Wu Y, Wu Z, Liu E (2014) The effect of hydroquinone as an electrolyte additive on electrochemical performance of the polyaniline supercapacitor[J]. Mater Res Bull 50:303–306
Chen Y-C, Lin L-Y (2019) Investigating the redox behavior of activated carbon supercapacitors with hydroquinone and p-phenylenediamine dual redox additives in the electrolyte[J]. J Colloid Interf Sci 537:295–305
Jokar E, Shahrokhian S, Zad AI (2014) Electrochemical functionalization of graphene nanosheets with catechol derivatives as an effective method for preparation of highly performance supercapacitors[J]. Electrochim Acta 147:136–142
Chen C, Yu C, Fu X, Wang Z (2014) Synthesis of graphite oxide-wrapped CuO nanocomposites for electrocatalytic oxidation of glucose[J]. Synth React Inorg, Met-Org, Nano-Met Chem 44(10):1521–1525
Funding
This work was financially supported by the Anhui Provincial Natural Science Foundation (Grant no.1908085MB37).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, K., Hua, L., Wang, Z. et al. One-step hydrothermal synthesis of reduced graphene oxide/carbon nanotubes/hydroquinone hydrogel and its electrochemical properties. Ionics 27, 5055–5065 (2021). https://doi.org/10.1007/s11581-021-04262-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04262-z