Abstract
Background
TAPUR is a pragmatic, phase II basket study evaluating the antitumor activity of commercially available targeted agents in patients with advanced cancers harboring genomic alterations known to be drug targets. Sunitinib is an oral multikinase inhibitor of FMS-like tyrosine kinase-3 (FLT-3), among other targets. Results from a cohort of patients with metastatic colorectal cancer (mCRC) with FLT-3 amplification treated with sunitinib are reported.
Objective
This study aimed to investigate whether patients with mCRC with FLT-3 amplification would be responsive to sunitinib, an oral multikinase inhibitor.
Methods
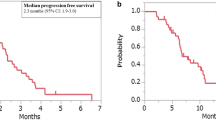
Eligible patients received a standard sunitinib dose of 50 mg orally for 4 weeks followed by 2 weeks off. Simon’s two-stage design was used with the primary study endpoint of objective response (OR) or stable disease (SD) at 16 weeks based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Secondary endpoints were progression-free survival, overall survival, and safety.
Results
Ten patients were enrolled from November 2016 to April 2018. All patients had mCRC with FLT-3 amplification. No ORs were observed. Although two patients had SD at 16 weeks, one died because of disease progression shortly thereafter and the cohort was closed. A single grade 3 adverse event of diarrhea was reported as possibly related to sunitinib.
Conclusions
Monotherapy with sunitinib does not have clinical activity in patients with mCRC with FLT-3 amplification and should not be prescribed for off-label use. Other treatments should be considered for these patients, including treatments offered in clinical trials.
Clinical Trial registration
NCT02693535 (26 February 2016).


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA A Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
National Cancer Institute. SEER Cancer Stat Facts: Colorectal Cancer. Bethesda, MD. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed 21 Feb 2020.
Tomlinson JS, Jarnagin WR, Dematteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–80. https://doi.org/10.1200/jco.2007.11.0833.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. https://doi.org/10.1056/nejm200104053441401.
Pacheco JM, Gao D, Smith D, Purcell T, Hancock M, Bunn P, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691–700. https://doi.org/10.1016/j.jtho.2018.12.014.
Langer CJ. The “Lazarus Response” in treatment-naïve, poor performance status patients with non–small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol. 2009;27(9):1350–4. https://doi.org/10.1200/jco.2008.20.4859.
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, De Braud F, Larkin J, et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N Engl J Med. 2014;371(20):1877–88. https://doi.org/10.1056/nejmoa1406037.
Bachtiar M, Ooi BNS, Wang J, Jin Y, Tan TW, Chong SS, et al. Towards precision medicine: interrogating the human genome to identify drug pathways associated with potentially functional, population-differentiated polymorphisms. Pharmacogenomics J. 2019;19(6):516–27. https://doi.org/10.1038/s41397-019-0096-y.
Mangat PK, Halabi S, Bruinooge SS, Garrett-Mayer E, Alva A, Janeway KA, et al. Rationale and design of the targeted agent and profiling utilization registry study. JCO Precis Oncol. 2018;2:1–14. https://doi.org/10.1200/po.18.00122.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon Alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. https://doi.org/10.1056/nejmoa065044.
Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–54. https://doi.org/10.1056/nejmoa1611406.
Demetri GD, Van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–38. https://doi.org/10.1016/s0140-6736(06)69446-4.
Raymond E, Dahan L, Raoul J-L, Bang Y-J, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–13.
Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25(30):4793–9. https://doi.org/10.1200/jco.2007.12.8637.
Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, Phase III trial. J Clin Oncol. 2013;31(10):1341–7. https://doi.org/10.1200/jco.2012.45.1930.
Moreira RB, Peixoto RDA, de Sousa Cruz MR. Clinical response to sorafenib in a patient with metastatic colorectal cancer and FLT3 amplification. Case Rep Oncol. 2015;8(1):83–7. https://doi.org/10.1159/000375483.
Al Baghdadi T, Halabi S, Garrett-Mayer E, Mangat PK, Ahn ER, Sahai V, et al. Palbociclib in patients with pancreatic and biliary cancer with CDKN2A alterations: results from the targeted agent and profiling utilization registry study. JCO Precis Oncol. 2019;3:1–8. https://doi.org/10.1200/po.19.00124.
Berenstein R. Class III Receptor Tyrosine Kinases in Acute Leukemia—Biological Functions and Modern Laboratory Analysis. Biomarker Insights. 2015;10s3:BMI.S22433. https://doi.org/10.4137/bmi.s22433.
Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol. 2013;97(6):683–94. https://doi.org/10.1007/s12185-013-1334-8.
Rosnet O, Bühring HJ, deLapeyrière O, Beslu N, Lavagna C, Marchetto S, et al. Expression and signal transduction of the FLT3 tyrosine kinase receptor. Acta Haematol. 1996;95(3–4):218–23. https://doi.org/10.1159/000203881.
Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42. https://doi.org/10.1182/blood-2002-02-0492.
Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87(3):1089–96.
Sutamtewagul G, Vigil CE. Clinical use of FLT3 inhibitors in acute myeloid leukemia. OncoTargets Therapy. 2018;11:7041–52. https://doi.org/10.2147/ott.s171640.
Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3(9):650–65. https://doi.org/10.1038/nrc1169.
Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16(6):991–1001. https://doi.org/10.1158/1535-7163.mct-16-0876.
O’Farrell A-M, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, et al. An innovative phase i clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465–76.
Fiedler W, Serve H, DöHner H, Schwittay M, Ottmann OG, O’Farrell A-M, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93. https://doi.org/10.1182/blood-2004-05-1846.
Fiedler W, Kayser S, Kebenko M, Janning M, Krauter J, Schittenhelm M, et al. A phase I/II study of sunitinib and intensive chemotherapy in patients over 60 years of age with acute myeloid leukaemia and activating FLT3 mutations. Br J Haematol. 2015;169(5):694–700. https://doi.org/10.1111/bjh.13353.
Lim SH, Kim S-Y, Kim K, Jang H, Ahn S, Kim K-M et al. The implication of FLT3 amplification for FLT targeted therapeutics in solid tumors. Oncotarget. 2017;8(2):3237–45. https://doi.org/10.18632/oncotarget.13700.
Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518(7539):422–6. https://doi.org/10.1038/nature13952.
Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–70. https://doi.org/10.1038/nature11252.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data: figure 1. Cancer Discov. 2012;2(5):401–4. https://doi.org/10.1158/2159-8290.cd-12-0095.
Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. https://doi.org/10.1038/nature13385.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795–801. https://doi.org/10.1038/nm.3870.
Braxton DR, Zhang R, Morrissette JD, Loaiza-Bonilla A, Furth EE. Clinicopathogenomic analysis of mismatch repair proficient colorectal adenocarcinoma uncovers novel prognostic subgroups with differing patterns of genetic evolution. Int J Cancer. 2016;139(7):1546–56. https://doi.org/10.1002/ijc.30196.
Hasegawa H, Taniguchi H, Kato T, Fujii S, Ebi H, Shiozawa M et al. Prognostic and predictive impact on FMS-like tyrosine kinase 3 (FLT3) amplification in patients with metastatic colorectal cancer. Annals of Oncology. 2019;30(Supplement_5):v240. https://doi.org/10.1093/annonc/mdz246.113.
Chow LQM, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–96. https://doi.org/10.1200/jco.2006.06.3602.
O’Farrell A-M, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KWH, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–605. https://doi.org/10.1182/blood-2002-07-2307.
Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–32. https://doi.org/10.1038/nbt1358.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64. https://doi.org/10.1056/nejmoa1614359.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–40.
Ponnurangam S, Standing D, Rangarajan P, Subramaniam D. Tandutinib Inhibits the Akt/mTOR Signaling Pathway to Inhibit Colon Cancer Growth. 2013;12(5):598–609. https://doi.org/10.1158/1535-7163.mct-12-0907.
Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. https://doi.org/10.1038/nm.3967.
Liu Y, Yang EJ, Zhang B, Miao Z, Wu C, Lyu J, et al. PTEN deficiency confers colorectal cancer cell resistance to dual inhibitors of FLT3 and aurora kinase A. Cancer Lett. 2018;436:28–37. https://doi.org/10.1016/j.canlet.2018.08.011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Sunitinib (Sutent) and funding for this study were provided by Pfizer, Inc. Additional funding was provided by AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Genentech, and Merck.
Conflict of interest
ASCO receives research support for the TAPUR Study from the following companies: AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Eli Lilly, Merck, and Pfizer. Susan Halabi, PhD, receives partial grant support from ASCO TAPUR. Seungjean Chai, MD, has participated in a consulting day with Cardinal Health. Tareq Al Baghdadi, MD; Elizabeth Garrett-Mayer, PhD; Pam K. Mangat, MS; Patricia Rich, MD; Eugene R. Ahn, MD; Andrew L. Rygiel, MPH; Olufunlayo Osayameh, MPH; Kaitlyn R. Antonelli; Samiha Islam; Suanna S. Bruinooge, MPH; and Richard L. Schilsky, MD have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by a central institutional review board and, in some cases, by a local institutional review board at participating sites.
Consent to participate
Patients provided written consent before engaging in study-related activities or providing study data and signed informed consent regarding publication of their data.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
TAB, EG-M, PKM, SSB, and RLS contributed to the conception and design of this manuscript. TAB, PR, ERA, and SC provided study materials. EG-M, PKM, ALR, OO, KRA, and SI collected and/or assembled data. TAB, EG-M, PKM, ALR, and RLS analyzed and interpreted the data. TAB, PR, EG-M, SH, PKM, OO, KRA, and RLS contributed to the writing and/or revision of the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al Baghdadi, T., Garrett-Mayer, E., Halabi, S. et al. Sunitinib in Patients with Metastatic Colorectal Cancer (mCRC) with FLT-3 Amplification: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. Targ Oncol 15, 743–750 (2020). https://doi.org/10.1007/s11523-020-00752-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00752-8