Abstract
Background
Golgi phosphoprotein 2 (GOLPH2) has been shown to be involved in chronic inflammatory processes and carcinogenesis. GOLPH2 is prominently overexpressed in hepatocellular carcinoma, melanoma, glioblastoma, prostate, lung, and colorectal cancer. With a low and tightly regulated expression in non-malignant tissues, GOLPH2 has been proposed as an attractive target for cancer therapy. However, GOLPH2 is predominantly located intracellularly and when situated outside of the cell it is proteolytically cleaved and shed from the cell surface. Until now, GOLPH2 has been regarded as an “undruggable” target.
Objective
We sought to create antibodies that specifically bind to GOLPH2 overexpressing tumor cells.
Patients and Methods
Antibodies binding to membranous GOLPH2 despite shedding of the protein were generated from a scFV library screening. These antibodies target the part of GOLPH2 that remains at the cell surface after proteolytic cleavage. These antibodies were then tested in vitro and in vivo.
Results
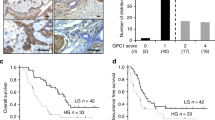
Two candidates (G2-1 and G2-2) showed target specific binding in vitro. Utilizing a tumor array (n = 128 tumors) with G2-2 and a reference antibody, a GOLPH2 expression scoring system was established. Rapid internalization of the antibodies was noted so this was exploited to deliver a toxic payload of pyrrolobenzodiazepine (PBD). In two patient-derived xenograft (PDX)-models, colorectal and lung cancer, the G2-2 antibody drug conjugate (ADC) displayed high efficacy with significant tumor responses (P = 0.001; P = 0.013) and improved survival (P = 0.0001; P = 0.0011) compared with controls.
Conclusions
Treatment with GOLPH2-directed antibodies induces durable responses in colorectal and lung cancer models. With a robust companion assay for GOLPH2 positivity at hand our findings prepare for the translation into a clinical trial.





Similar content being viewed by others
Abbreviations
- RTK:
-
Receptor tyrosine kinase
- GOLPH2:
-
Golgi Phosphoprotein 2
- mABs:
-
Monoclonal antibodies
- ADC:
-
Antibody drug conjugate
- FACS:
-
Fluorescence activated cell sorting
- IHC:
-
Immunohistochemistry
- IF:
-
Immunofluorescence
References
Zhang F, Gu Y, Li X, Wang W, He J, Peng T. Up-regulated Golgi phosphoprotein 2 (GOLPH2) expression in lung adenocarcinoma tissue. Clin Biochem. Can Soc Clin Chem [Internet]. 2010;43:983–91. https://doi.org/10.1016/j.clinbiochem.2010.05.010.
Donizy P, Kaczorowski M, Biecek P, Halon A, Szkudlarek T, Matkowski R. Golgi-related proteins GOLPH2 (GP73/GOLM1) and GOLPH3 (GOPP1/MIDAS) in cutaneous melanoma: patterns of expression and prognostic significance. Int J Mol Sci. 2016;17:1619. https://doi.org/10.3390/ijms17101619.
Kristiansen G, Fritzsche FR, Wassermann K, Jäger C, Tölle A, Lein M, et al. GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: implications for tissue-based diagnostics. Br J Cancer [Internet]. 2008;99:939–48. https://doi.org/10.1038/sj.bjc.6604614.
Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, et al. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49:1602–9. https://doi.org/10.1002/hep.22843.
Liu G, Zhang Y, He F, Li J, Wei X, Li Y, et al. Expression of GOLPH2 is associated with the progression of and poor prognosis in gastric cancer. Oncol Rep. 2014;32:2077–85. https://doi.org/10.3892/or.2014.3404.
Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–93. https://doi.org/10.1136/gut.2010.214916.
Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–12. https://doi.org/10.1016/j.jhep.2005.05.028.
Jiang K, Li W, Shang S, Sun L, Guo K, Zhang S, et al. Aberrant expression of Golgi protein 73 is indicative of a poor outcome in hepatocellular carcinoma. Oncol Rep [Internet]. 2016;35:2141–50. https://doi.org/10.3892/or.2016.4601.
Wei S, Dunn TA, Isaacs WB, De Marzo AM, Luo J. GOLPH2 and MYO6: putative prostate cancer markers localized to the golgi apparatus. Prostate. 2008;68(13):1387–95. https://doi.org/10.1002/pros.20806.
Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68(3):645–9. https://doi.org/10.1158/0008-5472.CAN-07-3224.
Liu X, Chen L, Zhang T. Increased GOLM1 expression independently predicts unfavorable overall survival and recurrence-free survival in lung adenocarcinoma. Cancer Control [Internet]. 2018;25:107327481877800. https://doi.org/10.1177/1073274818778001.
Zhang Y, Hu W, Wang L, Han B, Lin R, Wei N. Association of GOLPH2 expression with survival in non-small-cell lung cancer: clinical implications and biological validation. Biomark Med [Internet]. 2017;11:967–77. https://doi.org/10.2217/bmm-2017-0199.
El-zefzafy WM, Abu-zahab Z, Abdelwahab MA, Ahmed LI. Study of serum Dickkopf-1, and Golgi membrane protein in egyptian patients with colorectal cancer study of serum Dickkopf-1, and Golgi membrane protein in Egyptian patients with colorectal cancer. Clin Med Diagn. 2015;5(5):81–9. https://doi.org/10.5923/j.cmd.20150505.01.
Xu R, Ji J, Zhang X, Han M, Zhang C, Xu Y, et al. PDGFA/PDGFRα-regulated GOLM1 promotes human glioma progression through activation of AKT. J Exp Clin Cancer Res. 2017;36:1–17. https://doi.org/10.1186/s13046-017-0665-3.
Zhang W, Kim H, Lv J, Zhao N, Ma X. Golgi phosphoprotein 2 is a novel regulator of IL-12 production and macrophage polarization. J Immunol [Internet]. 2018;200:ji1700897. https://doi.org/10.4049/jimmunol.1700897.
Ye QH, Zhu WW, Zhang JB, Qin Y, Lu M, Lin GL, et al. GOLM1 modulates EGFR/RTK cell-surface recycling to drive hepatocellular carcinoma metastasis. Cancer Cell. 2016;30:444–58. https://doi.org/10.1016/j.ccell.2016.07.017.
Yang H-J, Liu G-L, Liu B, Liu T. GP73 promotes invasion and metastasis of bladder cancer by regulating the epithelial-mesenchymal transition through the TGF-β1/Smad2 signalling pathway. J Cell Mol Med [Internet]. 2018;22:1650–65. https://doi.org/10.1111/jcmm.13442
Yang Y, Liu Q, Zhang H, Zhao H, Mao R, Li Z, et al. Silencing of GP73 inhibits invasion and metastasis via suppression of epithelial–mesenchymal transition in hepatocellular carcinoma. Oncol Rep [Internet]. 2017. https://doi.org/10.3892/or.2017.5351
Liu Y, Zhang X, Zhou S, Shi J, Xu Y, He J, et al. Knockdown of Golgi phosphoprotein 73 blocks the trafficking of matrix metalloproteinase-2 in hepatocellular carcinoma cells and inhibits cell invasion. J Cell Mol Med [Internet]. 2019;23:2399–409. https://doi.org/10.1111/jcmm.14055.
Yan G, Ru Y, Wu K, Yan F, Wang Q, Wang J, et al. GOLM1 promotes prostate cancer progression through activating PI3K-AKT-mTOR signaling. Prostate [Internet]. 2017. https://doi.org/10.1002/pros.23461.
Chen X, Wang Y, Tao J, Shi Y, Gai X, Huang F, et al. MTORC1 Up-regulates GP73 to promote proliferation and migration of hepatocellular carcinoma cells and growth of xenograft tumors in mice (8A10!). Gastroenterology [Internet]. 2015;149:741–52. https://doi.org/10.1053/j.gastro.2015.05.005.
Kuang Z, Huang R, Yang Z, Lv Z, Chen X, Xu F, et al. Quantitative screening of serum protein biomarkers by reverse phase protein arrays. Oncotarget [Internet]. 2018;9:32624–41. https://doi.org/10.18632/oncotarget.25976.
Ye J-Z, Yan S, Yuan C, Wu H, Zhang J, Liu Z-H, et al. GP73 level determines chemotherapeutic resistance in human hepatocellular carcinoma cells. J Cancer [Internet]. 2018;9:415–23. https://doi.org/10.7150/jca.19185.
Nunes JPM, Vassileva V, Robinson E, Morais M, Smith MEB, Pedley RB, et al. Use of a next generation maleimide in combination with THIOMAB™ antibody technology delivers a highly stable, potent and near homogeneous THIOMAB™ antibody-drug conjugate (TDC). RSC Adv. 2017;7:24828–32. https://doi.org/10.1039/c7ra04606e.
Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, et al. A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci USA [Internet]. 2014;111:1766–71. https://doi.org/10.1073/pnas.1321237111.
Choppa PC, Vojdani A, Tagle C, Andrin R, Magtoto L. Multiplex PCR for the detection of Mycoplasma fermentans, M. hominis and M. penetrans in cell cultures and blood samples of patients with chronic fatigue syndrome. Mol Cell Probes [Internet]. 1998;12:301–8. https://doi.org/10.1006/mcpr.1998.0186.
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol [Internet]. 2003;21:778–84. https://doi.org/10.1038/nbt0803-941a.
Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem [Internet]. 2006;17:114–24. https://doi.org/10.1021/bc0502917.
Takahashi S, Kasai K, Hatsuzawa K, Kitamura N, Misumi Y, Ikehara Y, et al. A mutation of furin causes the lack of precursor-processing activity in human colon carcinoma LoVo cells. Biochem Biophys Res Commun [Internet]. 1993;195:1019–26. https://doi.org/10.1006/bbrc.1993.2146.
Acknowledgements
The authors thank the team at Cureab and the Cancer Immunology Laboratory Basel staff for their contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international and institutional guidelines for the care and use of animals were followed.
Funding
This study was funded by Cureab GmbH.
Conflict of interest
Authors HL and NM own stocks in Cureab GmbH. Author FS is on the advisory board of Cureab GmbH. CR has given uncompensated advice to Cureab GmbH. Authors HLae, YL, MM, NL, CR, and AZ declare that they have no conflicts of interest that might be relevant to the contents of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liewen, H., Markuly, N., Läubli, H. et al. Therapeutic Targeting of Golgi Phosphoprotein 2 (GOLPH2) with Armed Antibodies: A Preclinical Study of Anti-GOLPH2 Antibody Drug Conjugates in Lung and Colorectal Cancer Models of Patient Derived Xenografts (PDX). Targ Oncol 14, 577–590 (2019). https://doi.org/10.1007/s11523-019-00667-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00667-z