Abstract
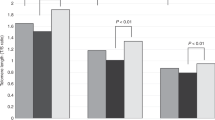
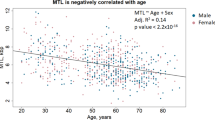
In humans, DNA methylation (DNAm) based estimators of telomere length (TL) have been shown to better predict TL-associated variables (e.g., age, sex, and mortality) than TL itself. The biological significance of DNAm-based estimators of TL (DNAmTL) is unclear. In vitro DNAmTL shortens with cell replications, even when telomerase is maintaining TL. Telomerase is typically suppressed in humans, except in testes. Accordingly, sperm TL increases with age, and offspring with greater paternal age at conception (PAC) have longer TL. Thus, we expect that PAC associations with DNAmTL can shed light on whether in vivo cell replications in the presence of high telomerase activity (production of sperm) shorten DNAmTL or if PAC-lengthened TL causes lengthened DNAmTL. In a pre-registered analysis, using data from 1733 blood samples from the Philippines, we examined the association between paternal age at conception (PAC) and offspring DNAmTL. We did not find an association between PAC and DNAmTL but found a positive association of paternal grandfather’s age at father’s conception predicting grandchild’s DNAmTL. In post hoc analyses, we examined how DNAmTL versus qPCR-measured TL (qPCR-TL) correlated with measures typically associated with TL. Contrary to previous findings, on almost all measures of external validity (correlations with parental TLs, southern blot TL, and age), qPCR-TL outperformed DNAmTL. The “kilobase” units of DNAm-based estimators of TL showed considerable deviations from southern blot-derived kilobase measures. Our findings suggest that DNAmTL is not a reliable index of inherited aspects of TL and underscores uncertainty about the biological meaning of DNAmTL.



Similar content being viewed by others
References
Lindrose AR, McLester-Davis LWY, Tristano RI, Kataria L, Gadalla SM, Eisenberg DTA, Verhulst S, Drury S. Method comparison studies of telomere length measurement using qPCR approaches: a critical appraisal of the literature. PLoS ONE. 2021;16(1): e0245582.
Lu AT, Seeboth A, Tsai PC, Sun D, Quach A, Reiner AP, et al. DNA methylation-based estimator of telomere length. Aging. 2019;11(16):5895–923.
Ryan CP. “Epigenetic clocks”: theory and applications in human biology. Am J Hum Biol. 2021;33(3): e23488.
Hastings WJ, Etzel L, Heim CM, Noll JG, Rose EJ, Schreier HM, Shenk CE, Tang X, Shalev I. Comparing qPCR and DNA methylation-based measurements of telomere length in a high-risk pediatric cohort. Aging. 2022;14(2):660.
Pearce EE, Horvath S, Katta S, Dagnall C, Aubert G, Hicks BD, Spellman SR, Katki H, Savage SA, Alsaggaf R. DNA-methylation-based telomere length estimator: comparisons with measurements from flow FISH and qPCR. Aging. 2021;13(11):14675.
Seki Y, Aczel D, Torma F, Jokai M, Boros A, Suzuki K, et al. No strong association among epigenetic modifications by DNA methylation, telomere length, and physical fitness in biological aging. Biogerontology. 2023;24(2):245–55.
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–9.
Eisenberg DTA, Kuzawa CW. The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences. 2018;373(1741).
Delgado DA, Zhang C, Gleason K, Demanelis K, Chen LS, Gao J, Roy S, Shinkle J, Sabarinathan M, Argos M, et al. The contribution of parent-to-offspring transmission of telomeres to the heritability of telomere length in humans. Hum Genet. 2019;138(1):49–60.
Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, Nanus DE, Budgeon CA, Musicha C, Bountziouka V, et al. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nature Aging. 2022;2(2):170–9.
Eisenberg DTA, Lee NR, Rej PH, Hayes MG, Kuzawa CW. Older paternal ages and grandpaternal ages at conception predict longer telomeres in human descendants. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1903):20190800.
Horvath K, Eisenberg D, Stone R, Anderson J, Kark J, Aviv A. Paternal age and transgenerational telomere length maintenance: a simulation model. Sci Rep. 2019;9(1):20.
Jenkins TG, Aston KI, Cairns B, Smith A, Carrell DT. Paternal germ line aging: DNA methylation age prediction from human sperm. BMC Genomics. 2018;19(1):763.
Jenkins TG, James ER, Aston KI, Salas-Huetos A, Pastuszak AW, Smith KR, Hanson HA, Hotaling JM, Carrell DT. Age-associated sperm DNA methylation patterns do not directly persist trans-generationally. Epigenetics Chromatin. 2019;12(1):74.
Kerepesi C, Zhang B, Lee S-G, Trapp A, Gladyshev VN. Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Science Advances. 2021;7(26):eabg6082.
Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci. 2012;109(26):10251–6.
Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40(3):619–25.
Kuzawa CW, Eisenberg DT. Intergenerational predictors of birth weight in the Philippines: correlations with mother’s and father’s birth weight and test of maternal constraint. PLoS ONE. 2012;7(7): e40905.
Eisenberg DT, Kuzawa CW, Hayes MG. Improving qPCR telomere length assays: controlling for well position effects increases statistical power. Am J Hum Biol. 2015;27(4):570–5.
Eisenberg DTA, Borja JB, Hayes MG, Kuzawa CW. Early life infection, but not breastfeeding, predicts adult blood telomere lengths in the Philippines. Am J Hum Biol. 2017;29(4): e22962.
Eisenberg DT. Telomere length measurement validity: the coefficient of variation is invalid and cannot be used to compare quantitative polymerase chain reaction and Southern blot telomere length measurement techniques. Int J Epidemiol. 2016;45(4):1295–8.
Verhulst S, Susser E, Factor-Litvak PR, Simons MJ, Benetos A, Steenstrup T, Kark JD, Aviv A. Commentary: the reliability of telomere length measurements. Int J Epidemiol. 2015;44(5):1683–6.
Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protocols. 2010;5(9):1596–607.
Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–9.
Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24(13):1547–8.
Higgins-Chen AT, Thrush KL, Wang Y, Minteer CJ, Kuo P-L, Wang M, Niimi P, Sturm G, Lin J, Moore AZ, et al. A computational solution for bolstering reliability of epigenetic clocks: implications for clinical trials and longitudinal tracking. Nature Aging. 2022;2(7):644–61.
Heiss JA, Just AC. Identifying mislabeled and contaminated DNA methylation microarray data: an extended quality control toolset with examples from GEO. Clin Epigenetics. 2018;10(1):73.
Stoffel MA, Nakagawa S, Schielzeth H. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol. 2017;8(11):1639–44.
Verhulst S. Improving comparability between qPCR-based telomere studies. Mol Ecol Resour. 2020;20(1):11–3.
Dahly DL, Adair LS. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban–rural dichotomy. Soc Sci Med. 2007;64(7):1407–19.
Eisenberg DTA, Rej PH, Duazo P, Carba D, Hayes MG, Kuzawa CW. Testing for paternal influences on offspring telomere length in a human cohort in the Philippines. Am J Phys Anthropol. 2020;171(3):520–8.
Rej PH, Tennyson RL, Lee NR, Eisenberg DTA. Years of caregiving for chronically ill and disabled family members is not associated with telomere length in the Philippines. Psychoneuroendocrinology. 2019;103:188–94.
Tennyson RL, Gettler LT, Kuzawa CW, Hayes MG, Agustin SS, Eisenberg DTA. Lifetime socioeconomic status and early life microbial environments predict adult blood telomere length in the Philippines. Am J Hum Biol. 2018;30(5): e23145.
Croteau-Chonka DC, Wu Y, Li Y, Fogarty MP, Lange LA, Kuzawa CW, McDade TW, Borja JB, Luo J, AbdelBaky O, et al. Population-specific coding variant underlies genome-wide association with adiponectin level. Hum Mol Genet. 2012;21(2):463–71.
Hellwege JN, Keaton JM, Giri A, Gao X, Velez Edwards DR, Edwards TL. Population Stratification in Genetic Association Studies. Curr Protoc Hum Genet. 2017;95(1):1.22.21-21.22.23.
Bethancourt HJ, Kratz M, Beresford SAA, Hayes MG, Kuzawa CW, Duazo PL, Borja JB, Eisenberg DTA. No association between blood telomere length and longitudinally assessed diet or adiposity in a young adult Filipino population. Eur J Nutr. 2017;56(1):295–308.
Heidinger BJ, Herborn KA, Granroth-Wilding HMV, Boner W, Burthe S, Newell M, Wanless S, Daunt F, Monaghan P. Parental age influences offspring telomere loss. Funct Ecol. 2016;30(9):1531–8.
Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry. 2011;168(1):82–8.
Bateson M, Eisenberg DTA, Nettle D. Controlling for baseline telomere length biases estimates of the rate of telomere attrition. Royal Society Open Science. 2019;6(10): 190937.
Nettle D, Gadalla SM, Lai T-P, Susser E, Bateson M, Aviv A. Measurement of telomere length for longitudinal analysis: implications of assay precision. Am J Epidemiol. 2021;190(7):1406–13.
Eisenberg DTA. An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23(2):149–67.
Knol MJ, Pestman WR, Grobbee DE. The (mis)use of overlap of confidence intervals to assess effect modification. Eur J Epidemiol. 2011;26(4):253–4.
Lindrose A, Drury S. Minimum reporting recommendations for PCR-based telomere length measurement. 2020. https://osf.io/preprints/osf/9pzst
Benetos A, Verhulst S, Labat C, Lai TP, Girerd N, Toupance S, Zannad F, Rossignol P, Aviv A. Telomere length tracking in children and their parents: implications for adult onset diseases. FASEB J. 2019;33(12):14248–53.
McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89(4):1237–45.
Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012;730(1–2):59–67.
Acknowledgements
Discussions with Daniel Belsky, Waylon Hastings, Idan Shalev, and Simon Verhulst at the 2021 Telomere Research Network meeting were helpful in refining and inspiring the ideas of this paper. Thanks to Michael Le Pepke for valuable feedback on a draft of this manuscript.
Funding
National Institutes of Health, Grant/Award Numbers: R01AG061006, DK056350, DK078150, ES10126, RR20649, TW05596; National Science Foundation, Grant/Award Numbers: BCS-0962282, BCS-1519110; Wenner-Gren Foundation, Grant/Award Number: 8111.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Eisenberg, D.T.A., Ryan, C., Lee, N.R. et al. DNA methylation-based estimators of telomere length show low correspondence with paternal age at conception and other measures of external validity of telomere length. GeroScience (2024). https://doi.org/10.1007/s11357-024-01114-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01114-2