Abstract
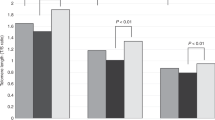
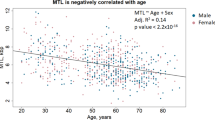
Leukocyte telomere length (LTL) is a heritable trait with two potential sources of heritability (h2): inherited variation in non-telomeric regions (e.g., SNPs that influence telomere maintenance) and variability in the lengths of telomeres in gametes that produce offspring zygotes (i.e., “direct” inheritance). Prior studies of LTL h2 have not attempted to disentangle these two sources. Here, we use a novel approach for detecting the direct inheritance of telomeres by studying the association between identity-by-descent (IBD) sharing at chromosome ends and phenotypic similarity in LTL. We measured genome-wide SNPs and LTL for a sample of 5069 Bangladeshi adults with substantial relatedness. For each of the 6318 relative pairs identified, we used SNPs near the telomeres to estimate the number of chromosome ends shared IBD, a proxy for the number of telomeres shared IBD (Tshared). We then estimated the association between Tshared and the squared pairwise difference in LTL ((ΔLTL)2) within various classes of relatives (siblings, avuncular, cousins, and distant), adjusting for overall genetic relatedness (ϕ). The association between Tshared and (ΔLTL)2 was inverse among all relative pair types. In a meta-analysis including all relative pairs (ϕ > 0.05), the association between Tshared and (ΔLTL)2 (P = 0.01) was stronger than the association between ϕ and (ΔLTL)2 (P = 0.43). Our results provide strong evidence that telomere length (TL) in parental germ cells impacts TL in offspring cells and contributes to LTL h2 despite telomere “reprogramming” during embryonic development. Applying our method to larger studies will enable robust estimation of LTL h2 attributable to direct transmission of telomeres.





Similar content being viewed by others
References
Achi MV, Ravindranath N, Dym M (2000) Telomere length in male germ cells is inversely correlated with telomerase activity. Biol Reprod 63:591–598
Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen A, Howe G, Graziano J (2005) Health effects of arsenic longitudinal study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol 16:191–205
Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 89:10114–10118
Argos M, Rahman M, Parvez F, Dignam J, Islam T, Quasem I, Hore SK, Haider AT, Hossain Z, Patwary TI et al (2013) Baseline comorbidities in a skin cancer prevention trial in Bangladesh. Eur J Clin Investig 43:579–588
Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88:557–579
Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM (2012) Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLOS Genet 8:e1002696
Bischoff C, Graakjaer J, Petersen HC, Jeune B, Bohr VA, Koelvraa S, Christensen K (2012) Telomere length among the elderly and oldest-old. Twin Res Hum Genet 8:425–432
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350:1193–1198
Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, de Geus EJC et al (2013a) Meta-analysis of telomere length in 19[thinsp]713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet 21:1163–1168
Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, de Geus EJC et al (2013b) Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet 21:1163–1168
Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, Compton CC, DeLuca DS, Peter-Demchok J, Gelfand ET et al (2015) A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobank 13:311–319
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47–e47
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Chiang YJ, Calado RT, Hathcock KS, Lansdorp PM, Young NS, Hodes RJ (2010) Telomere length is inherited with resetting of the telomere set-point. Proc Natl Acad Sci 107:10148–10153
Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N et al (2010) Common variants near TERC are associated with mean telomere length. Nat Genet 42:197–199
Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I et al (2013) Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 45:422–427
Collopy LC, Walne AJ, Cardoso S, de la Fuente J, Mohamed M, Toriello H, Tamary H, Ling AJYV, Lloyd T, Kassam R et al (2015) Triallelic and epigenetic-like inheritance in human disorders of telomerase. Blood 126:176
De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Criekinge W, De Backer GG, Gillebert TC, Van Oostveldt P, Bekaert S (2007) Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet 16:3097–3102
De Meyer T, Vandepitte K, Denil S, De Buyzere ML, Rietzschel ER, Bekaert S (2014) A non-genetic, epigenetic-like mechanism of telomere length inheritance? Eur J Hum Genet 22:10–11
Delgado DA, Zhang C, Chen LS, Gao J, Roy S, Shinkle J, Sabarinathan M, Argos M, Tong L, Ahmed A et al (2017) Genome-wide association study of telomere length among South Asians identifies a second RTEL1 association signal. J Med Genet 55:64–71
Do SK, Yoo SS, Choi YY, Choi JE, Jeon H-S, Lee WK, Lee SY, Lee J, Cha SI, Kim CH et al (2015) Replication of the results of genome-wide and candidate gene association studies on telomere length in a Korean population. Korean J Intern Med 30:719–726
eGTEx Project (2017) Enhancing GTEx by bridging the gaps between genotype, gene expression, and disease. Nat Genet 49:1664
Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstätter A (2009) Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol 38:1725–1734
Eisenberg DTA, Hayes MG, Kuzawa CW (2012) Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci 109:10251–10256
Faul JD, Mitchell CM, Smith JA, Zhao W (2016) Estimating telomere length heritability in an unrelated sample of adults: is heritability of telomere length modified by life course socioeconomic status? Biodemogr Soc Biol 62, 73–86
Hansen ME, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, Hirbo J, Beggs W, Reiner AP, Wilson JG et al (2016) Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum Mol Genet 25:2324–2330
Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. Br Med J 349:g4227
Hjelmborg JB, Dalgård C, Möller S, Steenstrup T, Kimura M, Christensen K, Kyvik KO, Aviv A (2015) The heritability of leucocyte telomere length dynamics. J Med Genet 52:297–302
Honig LS, Kang MS, Cheng R, Eckfeldt JH, Thyagarajan B, Leiendecker-Foster C, Province MA, Sanders JL, Perls T, Christensen K et al (2015) Heritability of telomere length in a study of long-lived families. Neurobiol Aging 36:2785–2790
Huang Y, Liang P, Liu D, Huang J, Songyang Z (2014) Telomere regulation in pluripotent stem cells. Protein Cell 5:194–202
Iles MM, Bishop DT, Taylor JC, Hayward NK, Brossard M, Cust AE, Dunning AM, Lee JE, Moses EK, Akslen LA et al (2014) The effect on melanoma risk of genes previously associated with telomere length. J Natl Cancer Inst 106:dju267
Kalmbach K, Robinson LG Jr, Wang F, Liu L, Keefe D (2014) Telomere length reprogramming in embryos and stem cells. Biomed Res Int 2014:925121
Kalmbach KH, Antunes DMF, Dracxler RC, Knier TW, Seth-Smith ML, Wang F, Liu L, Keefe DL (2013) Telomeres and human reproduction. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2012.1011.1039
Kibriya MG, Jasmine F, Roy S, Ahsan H, Pierce B (2014) Measurement of telomere length: a new assay using QuantiGene chemistry on a Luminex platform. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cospons Am Soc Prev Oncol 23:2667–2672
Kibriya MG, Jasmine F, Roy S, Ahsan H, Pierce BL (2016) Novel luminex assay for telomere repeat mass does not show well position effects like qPCR. PloS One 11:e0155548
Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, Cupples A, Hunkin JL, Gardner JP, Lu X et al (2008) Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLOS Genet 4:e37
Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang S-J, Chen W, Bis JC, Fitzpatrick AL, Smith E, Johnson AD et al (2010) Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci USA 107:9293–9298
Liu L, Trimarchi JR, Smith PJ, Keefe DL (2002) Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1:40–46
Mangino M, Hwang S-J, Spector TD, Hunt SC, Kimura M, Fitzpatrick AL, Christiansen L, Petersen I, Elbers CC, Harris T et al (2012) Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet 21:5385–5394
Mangino M, Christiansen L, Stone R, Hunt SC, Horvath K, Eisenberg DTA, Kimura M, Petersen I, Kark JD, Herbig U et al (2015) DCAF4, a novel gene associated with leucocyte telomere length. J Med Genet 52:157–162
Ojha J, Codd V, Nelson CP, Samani NJ, Smirnov IV, Madsen NR, Hansen HM, de Smith AJ, Bracci PM, Wiencke JK et al (2016) Genetic variation associated with longer telomere length increases risk of chronic lymphocytic leukemia. Cancer Epidemiol Biomark Prev 25:1043–1049
Pierce BL, Kibriya MG, Tong L, Jasmine F, Argos M, Roy S, Paul-Brutus R, Rahaman R, Rakibuz-Zaman M, Parvez F et al (2012) Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet 8:e1002522
Pierce BL, Tong L, Argos M, Gao J, Jasmine F, Roy S, Paul-Brutus R, Rahaman R, Rakibuz-Zaman M, Parvez F et al (2013) Arsenic metabolism efficiency has a causal role in arsenic toxicity: Mendelian randomization and gene–environment interaction. Int J Epidemiol 42:1862–1872
Pierce BL, Jasmine F, Roy S, Zhang C, Aviv A, Hunt SC, Ahsan H, Kibriya MG (2016) Telomere length measurement by a novel Luminex-based assay: a blinded comparison to Southern blot. Int J Mol Epidemiol Genet 7:18–23
Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, Michailidou K, Tyrer JP, Benlloch S, Brown J et al (2013) A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet 22:5056–5064
Prescott J, Kraft P, Chasman DI, Savage SA, Mirabello L, Berndt SI, Weissfeld JL, Han J, Hayes RB, Chanock SJ et al (2011) Genome-wide association study of relative telomere length. PloS One 6:e19635
Prescott J, Du M, Wong JY, Han J, De Vivo I (2012) Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum Reprod (Oxf Engl) 27:3622–3631
Shen Q, Zhang Z, Yu L, Cao L, Zhou D, Kan M, Li B, Zhang D, He L, Liu Y (2011) Common variants near TERC are associated with leukocyte telomere length in the Chinese Han population. Eur J Hum Genet 19:721–723
Soerensen M, Thinggaard M, Nygaard M, Dato S, Tan Q, Hjelmborg J, Andersen-Ranberg K, Stevnsner T, Bohr VA, Kimura M et al (2012) Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: a cross sectional and longitudinal analysis. Aging Cell 11:223–227
Stindl R (2016) The paradox of longer sperm telomeres in older men’s testes: a birth-cohort effect caused by transgenerational telomere erosion in the female germline. Mol Cytogenet 9:12
Unryn BM, Cook LS, Riabowol KT (2005) Paternal age is positively linked to telomere length of children. Aging Cell 4:97–101
Visscher PM, Medland SE, Ferreira MAR, Morley KI, Zhu G, Cornes BK, Montgomery GW, Martin NG (2006) Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLOS Genet 2:e41
Walsh KM, Codd V, Rice T, Nelson CP, Smirnov IV, McCoy LS, Hansen HM, Elhauge E, Ojha J, Francis SS et al (2015) Longer genotypically-estimated leukocyte telomere length is associated with increased adult glioma risk. Oncotarget 6:42468–42477
Walsh KM, Whitehead TP, de Smith AJ, Smirnov IV, Park M, Endicott AA, Francis SS, Codd V, Samani NJ, Metayer C et al (2016) Common genetic variants associated with telomere length confer risk for neuroblastoma and other childhood cancers. Carcinogenesis 37:576–582
Willeit P, Willeit J, Brandstätter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F et al (2010a) Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 30:1649–1656
Willeit P, Willeit J, Mayr A et al (2010b) Telomere length and risk of incident cancer and cancer mortality. JAMA 304:69–75
Zhang C, Doherty JA, Burgess S, Hung RJ, Lindström S, Kraft P, Gong J, Amos CI, Sellers TA, Monteiro ANA et al (2015) Genetic determinants of telomere length and risk of common cancers: a Mendelian randomization study. Hum Mol Genet 24:5356–5366
Zhang C, Kibriya MG, Jasmine F, Roy S, Gao J, Sabarinathan M, Shinkle J, Delgado D, Ahmed A, Islam T et al (2018) A study of telomere length, arsenic exposure, and arsenic toxicity in a Bangladeshi cohort. Environ Res 164:346–355
Acknowledgements
This work was supported by the National Institutes of Health (R01 ES020506, U01 HG007601, P42 ES10349, R01 CA107431, R01 CA102484, P30 CA014599). The authors thank all the men and women who participated in HEALS and BEST and all research staff who contributed to data collection. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Delgado, D.A., Zhang, C., Gleason, K. et al. The contribution of parent-to-offspring transmission of telomeres to the heritability of telomere length in humans. Hum Genet 138, 49–60 (2019). https://doi.org/10.1007/s00439-018-1964-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-018-1964-2