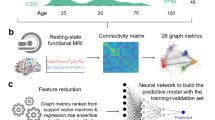
Abstract
The effects of age and gender on large-scale resting-state networks (RSNs) reflecting within- and between-network connectivity in the healthy brain remain unclear. This study investigated how age and gender influence the brain network roles and topological properties underlying the ageing process. Ten RSNs were constructed based on 998 participants from the REST-meta-MDD cohort. Multivariate linear regression analysis was used to examine the independent and interactive influences of age and gender on large-scale RSNs and their topological properties. A support vector regression model integrating whole-brain network features was used to predict brain age across the lifespan and cognitive decline in an Alzheimer’s disease spectrum (ADS) sample. Differential effects of age and gender on brain network roles were demonstrated across the lifespan. Specifically, cingulo-opercular, auditory, and visual (VIS) networks showed more incohesive features reflected by decreased intra-network connectivity with ageing. Further, females displayed distinctive brain network trajectory patterns in middle-early age, showing enhanced network connectivity within the fronto-parietal network (FPN) and salience network (SAN) and weakened network connectivity between the FPN-somatomotor, FPN-VIS, and SAN-VIS networks. Age — but not gender — induced widespread decrease in topological properties of brain networks. Importantly, these differential network features predicted brain age and cognitive impairment in the ADS sample. By showing that age and gender exert specific dispersion of dynamic network roles and trajectories across the lifespan, this study has expanded our understanding of age- and gender-related brain changes with ageing. Moreover, the findings may be useful for detecting early-stage dementia.




Similar content being viewed by others
References
Renteria ME, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry. 2017;7:e1116. https://doi.org/10.1038/tp.2017.84.
Li LW, Ding D, Wu B, Dong X. Change of Cognitive Function in U.S. Chinese Older Adults: A Population-Based Study. J Gerontol A Biol Sci Med Sci. 2017;72:S5–10. https://doi.org/10.1093/gerona/glx004.
Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37:384–400. https://doi.org/10.1016/j.neubiorev.2013.01.017.
Konar A, Singh P, Thakur MK. Age-associated Cognitive Decline: Insights into Molecular Switches and Recovery Avenues. Aging Dis. 2016;7:121–9. https://doi.org/10.14336/AD.2015.1004.
Yu J, et al. Differences between multimodal brain-age and chronological-age are linked to telomere shortening. Neurobiol Aging. 2022;115:60–9. https://doi.org/10.1016/j.neurobiolaging.2022.03.015.
Luo N, et al. Age-related structural and functional variations in 5,967 individuals across the adult lifespan. Hum Brain Mapp. 2020;41:1725–37. https://doi.org/10.1002/hbm.24905.
Staffaroni AM, et al. The Longitudinal Trajectory of Default Mode Network Connectivity in Healthy Older Adults Varies As a Function of Age and Is Associated with Changes in Episodic Memory and Processing Speed. J Neurosci. 2018;38:2809–17. https://doi.org/10.1523/JNEUROSCI.3067-17.2018.
Muller-Oehring EM, et al. Influences of Age, Sex, and Moderate Alcohol Drinking on the Intrinsic Functional Architecture of Adolescent Brains. Cereb Cortex. 2018;28:1049–63. https://doi.org/10.1093/cercor/bhx014.
Allen EA, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. https://doi.org/10.3389/fnsys.2011.00002.
Huang CC, et al. Age-related changes in resting-state networks of a large sample size of healthy elderly. CNS Neurosci Ther. 2015;21:817–25. https://doi.org/10.1111/cns.12396.
Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17(471):549–458. https://doi.org/10.1038/mp.2011.81.
Cui Z, et al. Individual Variation in Functional Topography of Association Networks in Youth. Neuron. 2020;106:340-353.e348. https://doi.org/10.1016/j.neuron.2020.01.029.
Edde M, Leroux G, Altena E, Chanraud S. Functional brain connectivity changes across the human life span: From fetal development to old age. J Neurosci Res. 2021;99:236–62. https://doi.org/10.1002/jnr.24669.
Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage. 2017;160:32–40. https://doi.org/10.1016/j.neuroimage.2017.01.077.
Jahanshad N, Thompson PM. Multimodal neuroimaging of male and female brain structure in health and disease across the life span. J Neurosci Res. 2017;95:371–9. https://doi.org/10.1002/jnr.23919.
Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–68. https://doi.org/10.1016/j.neuroimage.2013.12.044.
Gur RC, Gur RE. Complementarity of sex differences in brain and behavior: From laterality to multimodal neuroimaging. J Neurosci Res. 2017;95:189–99. https://doi.org/10.1002/jnr.23830.
Nebli A, Rekik I. Gender differences in cortical morphological networks. Brain Imaging Behav. 2020;14:1831–9. https://doi.org/10.1007/s11682-019-00123-6.
Nebli A, Rekik I. Adversarial brain multiplex prediction from a single brain network with application to gender fingerprinting. Med Image Anal. 2021;67:101843. https://doi.org/10.1016/j.media.2020.101843.
Shanmugan S, et al. Sex differences in the functional topography of association networks in youth. Proc Natl Acad Sci U S A. 2022;119:e2110416119. https://doi.org/10.1073/pnas.2110416119.
Rubin LH, et al. Sex differences in associations of arginine vasopressin and oxytocin with resting-state functional brain connectivity. J Neurosci Res. 2017;95:576–86. https://doi.org/10.1002/jnr.23820.
Li H, Satterthwaite TD, Fan Y. Brain Age Prediction Based on Resting-State Functional Connectivity Patterns Using Convolutional Neural Networks. Proc IEEE Int Symp Biomed Imaging. 2018;101–104:2018. https://doi.org/10.1109/ISBI.2018.8363532.
Liem F, et al. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–88. https://doi.org/10.1016/j.neuroimage.2016.11.005.
Yan CG, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U S A. 2019;116:9078–83. https://doi.org/10.1073/pnas.1900390116.
Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111:E4997-5006. https://doi.org/10.1073/pnas.1415122111.
Yan C, Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13.
Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-Related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55. https://doi.org/10.1002/mrm.1910350312.
Lydon-Staley DM, Ciric R, Satterthwaite TD, Bassett DS. Evaluation of confound regression strategies for the mitigation of micromovement artifact in studies of dynamic resting-state functional connectivity and multilayer network modularity. Netw Neurosci. 2018;3:427–54. https://doi.org/10.1162/netn_a_00071.
Laumann TO, et al. On the Stability of BOLD fMRI Correlations. Cereb Cortex. 2017;27:4719–32. https://doi.org/10.1093/cercor/bhw265.
Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage. 2002;17:825–41. https://doi.org/10.1006/nimg.2002.1132.
Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. https://doi.org/10.1016/j.neuron.2011.09.006.
Cole MW, et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–55. https://doi.org/10.1038/nn.3470.
Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. https://doi.org/10.1371/journal.pcbi.0030017.
He Y, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–79. https://doi.org/10.1093/brain/awp089.
Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. https://doi.org/10.1103/PhysRevLett.87.198701.
Bethlehem RAI, et al. Dispersion of functional gradients across the adult lifespan. Neuroimage. 2020;222:117299. https://doi.org/10.1016/j.neuroimage.2020.117299.
Zonneveld HI, et al. Patterns of functional connectivity in an aging population: The Rotterdam Study. Neuroimage. 2019;189:432–44. https://doi.org/10.1016/j.neuroimage.2019.01.041.
Sheffield JM, et al. Transdiagnostic Associations Between Functional Brain Network Integrity and Cognition. JAMA Psychiat. 2017;74:605–13. https://doi.org/10.1001/jamapsychiatry.2017.0669.
Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016;41:159–72. https://doi.org/10.1016/j.neurobiolaging.2016.02.020.
Hausman HK, et al. The Role of Resting-State Network Functional Connectivity in Cognitive Aging. Front Aging Neurosci. 2020;12:177. https://doi.org/10.3389/fnagi.2020.00177.
Kuiper JJ, et al. A parcellation-based model of the auditory network. Hear Res. 2020;396:108078. https://doi.org/10.1016/j.heares.2020.108078.
Monge ZA, Stanley ML, Geib BR, Davis SW, Cabeza R. Functional networks underlying item and source memory: shared and distinct network components and age-related differences. Neurobiol Aging. 2018;69:140–50. https://doi.org/10.1016/j.neurobiolaging.2018.05.016.
Betzel RF, et al. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102(Pt 2):345–57. https://doi.org/10.1016/j.neuroimage.2014.07.067.
Park JE, et al. Differences in dynamic and static functional connectivity between young and elderly healthy adults. Neuroradiology. 2017;59:781–9. https://doi.org/10.1007/s00234-017-1875-2.
Wang Y, et al. Multimodal mapping of the face connectome. Nat Hum Behav. 2020;4:397–411. https://doi.org/10.1038/s41562-019-0811-3.
Monteiro TS, King BR, ZivariAdab H, Mantini D, Swinnen SP. Age-related differences in network flexibility and segregation at rest and during motor performance. Neuroimage. 2019;194:93–104. https://doi.org/10.1016/j.neuroimage.2019.03.015.
Archer JA, Lee A, Qiu A, Chen S-HA. A Comprehensive Analysis of Connectivity and Aging Over the Adult Life Span. Brain Connect. 2015;6:169–85. https://doi.org/10.1089/brain.2015.0345.
La Corte V, et al. Cognitive Decline and Reorganization of Functional Connectivity in Healthy Aging: The Pivotal Role of the Salience Network in the Prediction of Age and Cognitive Performances. Front Aging Neurosci. 2016;8:204. https://doi.org/10.3389/fnagi.2016.00204.
Das M, Singh V, Uddin LQ, Banerjee A, Roy D. Reconfiguration of Directed Functional Connectivity Among Neurocognitive Networks with Aging: Considering the Role of Thalamo-Cortical Interactions. Cereb Cortex. 2020. https://doi.org/10.1093/cercor/bhaa334.
Chen Y, et al. Age-related early/late variations of functional connectivity across the human lifespan. Neuroradiology. 2018;60:403–12. https://doi.org/10.1007/s00234-017-1973-1.
Zhang C, et al. Sex and Age Effects of Functional Connectivity in Early Adulthood. Brain Connect. 2016;6:700–13. https://doi.org/10.1089/brain.2016.0429.
Rothwell ES, Workman KP, Wang D, Lacreuse A. Sex differences in cognitive aging: a 4-year longitudinal study in marmosets. Neurobiol Aging. 2021;109:88–99. https://doi.org/10.1016/j.neurobiolaging.2021.09.015.
Demetrius LA, Eckert A, Grimm A. Sex differences in Alzheimer’s disease: metabolic reprogramming and therapeutic intervention. Trends Endocrinol Metab. 2021;32:963–79. https://doi.org/10.1016/j.tem.2021.09.004.
Scheinost D, et al. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 2015;36:1524–35. https://doi.org/10.1002/hbm.22720.
Zuo XN, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–43. https://doi.org/10.1523/JNEUROSCI.2612-10.2010.
Graves WW, Coulanges L, Levinson H, Boukrina O, Conant LL. Neural Effects of Gender and Age Interact in Reading. Front Neurosci. 2019;13:1115. https://doi.org/10.3389/fnins.2019.01115.
Goldstone A, et al. Gender Specific Re-organization of Resting-State Networks in Older Age. Front Aging Neurosci. 2016;8:285. https://doi.org/10.3389/fnagi.2016.00285.
Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb Cortex. 2015;25:1987–99. https://doi.org/10.1093/cercor/bhu012.
Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016; 41:159–72. https://doi.org/10.1016/j.neurobiolaging.2016.02.020.
Hausman HK, et al. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. Geroscience. 2022;44:847–66. https://doi.org/10.1007/s11357-021-00503-1.
Gomez-Ramirez J, Li Y, Wu Q, Wu J. A Quantitative Study of Network Robustness in Resting-State fMRI in Young and Elder Adults. Front Aging Neurosci. 2015;7:256. https://doi.org/10.3389/fnagi.2015.00256.
Hou F, et al. Age-Related Alterations in Electroencephalography Connectivity and Network Topology During n-Back Working Memory Task. Front Hum Neurosci. 2018;12:484. https://doi.org/10.3389/fnhum.2018.00484.
Knyazev GG, Volf NV, Belousova LV. Age-related differences in electroencephalogram connectivity and network topology. Neurobiol Aging. 2015;36:1849–59. https://doi.org/10.1016/j.neurobiolaging.2015.02.007.
Chong JSX, et al. Longitudinal Changes in the Cerebral Cortex Functional Organization of Healthy Elderly. J Neurosci. 2019;39:5534–50. https://doi.org/10.1523/JNEUROSCI.1451-18.2019.
Gong G, et al. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009;29:15684–93. https://doi.org/10.1523/JNEUROSCI.2308-09.2009.
Franke K, Ziegler G, Kloppel S, Gaser C, Alzheimer’s Disease Neuroimaging, I. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50:883–92. https://doi.org/10.1016/j.neuroimage.2010.01.005.
Acknowledgements
We thank the Depression Imaging REsearch ConsorTium for sharing data. We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Consortium name: Depression Imaging REsearch ConsorTium
Chao-Gan Yan (Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China; Magnetic Resonance Imaging Research Center and Research Center for Lifespan Development of Mind and Brain, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Child and Adolescent Psychiatry, New York University School of Medicine, New York, US), Xiao Chen (Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China), Le Li (Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China), Francisco Xavier Castellanos (Department of Child and Adolescent Psychiatry, New York University School of Medicine, New York, US; Nathan Kline Institute for Psychiatric Research, Orangeburg, US), Tong-Jian Bai (Anhui Medical University, Anhui, China), Qi-Jing Bo (Beijing Anding Hospital, Capital Medical University, Beijing, China), Guan-Mao Chen (The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, China), Ning-Xuan Chen (Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China), Wei Chen (Department of Psychiatry, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China), Chang Cheng (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Yu-Qi Cheng (First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China), Xi-Long Cui (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Jia Duan (Department of Psychiatry, First Affiliated Hospital, China Medical University, Liaoning, China), Yi-Ru Fang (Department of Psychiatry, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Qi-Yong Gong (Department of Radiology, Huaxi MR Research Center, West China Hospital of Sichuan University, Sichuan, China; Psychoradiology Research Unit of Chinese Academy of Medical Sciences, West China Hospital of Sichuan University, Sichuan, China), Wen-Bin Guo (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Zheng-Hua Hou (Department of Psychosomatics and Psychiatry, Zhongda Hospital, School of Medicine, Southeast University, Jiangsu, China), Lan Hu (Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China), Li Kuang (Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China), Feng Li (Beijing Anding Hospital, Capital Medical University, Beijing 100,054, China), Kai-Ming Li (Department of Radiology, Huaxi MR Research Center, West China Hospital of Sichuan University, Sichuan, China), Tao Li (Mental Health Center, West China Hospital, Sichuan University, Sichuan, China), Yan-Song Liu (Department of Clinical Psychology, Suzhou Psychiatric Hospital, The Affiliated Guangji Hospital of Soochow University, Jiangsu, China), Zhe-Ning Liu (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Yi-Cheng Long (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Qing-Hua Luo (Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China), Hua-Qing Meng (Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China), Dai-Hui Peng (Department of Psychiatry, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Hai-Tang Qiu (Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China), Jiang Qiu (Faculty of Psychology, Southwest University, Chongqing, China), Yue-Di Shen (Department of Radiology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Zhejiang, China), Yu-Shu Shi (Xi’an Central Hospital, Shaanxi, China), Chuan-Yue Wang (Beijing Anding Hospital, Capital Medical University, Beijing, China), Fei Wang (Department of Psychiatry, First Affiliated Hospital, China Medical University, Liaoning, China), Kai Wang (Anhui Medical University, Anhui, China), Li Wang (National Clinical Research Center for Mental Disorders, Peking University Sixth Hospital, Beijing, China; Key Laboratory of Mental Health, Ministry of Health, Peking University, Beijing, China), Xiang Wang (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Ying Wang (The First Affiliated Hospital of Jinan University, Guangdong, China), Xiao-Ping Wu (Xi’an Central Hospital, Shaanxi, China), Xin-Ran Wu (Faculty of Psychology, Southwest University, Chongqing, China), Guang-Rong Xie (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Hai-Yan Xie (Department of Psychiatry, The Fourth Affiliated Hospital, College of Medicine, Zhejiang University, Zhejiang, China), Peng Xie (Institute of Neuroscience, Chongqing Medical University, Chongqing, China; Chongqing Key Laboratory of Neurobiology, Chongqing, China; and Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China), Xiu-Feng Xu (First Affiliated Hospital of Kunming Medical University, Yunnan, China), Hong Yang (Department of Radiology, the First Affiliated Hospital, College of Medicine, Zhejiang University, Zhejiang, China), Jian Yang (The First Affiliated Hospital of Xi’an Jiaotong University, Shanxi, China), Jia-Shu Yao (Department of Psychiatry, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Zhejiang, China), Shu-Qiao Yao (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Ying-Ying Yin (Department of Psychosomatics and Psychiatry, Zhongda Hospital, School of Medicine, Southeast University, Jiangsu, China), Yong-Gui Yuan (Department of Psychosomatics and Psychiatry, Zhongda Hospital, School of Medicine, Southeast University, Jiangsu, China), Ai-Xia Zhang (The First Affiliated Hospital of Xi’an Jiaotong University, Shanxi, China), Hong Zhang (Xi’an Central Hospital, Shaanxi, China), Ke-Rang Zhang (First Hospital of Shanxi Medical University, Shanxi, China), Lei Zhang (Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China), Ru-Bai Zhou (Department of Psychiatry, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Yi-Ting Zhou (Mental Health Center, West China Hospital, Sichuan University, Sichuan, China), Jun-Juan Zhu (Department of Psychiatry, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Chao-Jie Zou (First Affiliated Hospital of Kunming Medical University, Yunnan, China), Tian-Mei Si (National Clinical Research Center for Mental Disorders, Peking University Sixth Hospital, Beijing, China; and Key Laboratory of Mental Health, Ministry of Health, Peking University, Beijing, China), Xi-Nian Zuo (Key Laboratory of Behavioral Science, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China; and Magnetic Resonance Imaging Research Center and Research Center for Lifespan Development of Mind and Brain, Institute of Psychology, Chinese Academy of Sciences, Beijing, China), Jing-Ping Zhao (Department of Psychiatry, The Second Xiangya Hospital of Central South University, Wuhan, China), Yu-Feng Zang (Center for Cognition and Brain Disorders, Institutes of Psychological Sciences, Hangzhou Normal University, Zhejiang, China; and Zhejiang Key Laboratory for Research in Assessment of Cognitive Impairments, Zhejiang, China) and Chunming Xie (Department of Neurology, Affiliated ZhongDa Hospital, School of Medicine, Southeast University, Jiangsu, China; Institute of Neuropsychiatry, Affiliated ZhongDa Hospital, Southeast University, Jiangsu, China; and the Key Laboratory of Developmental Genes and Human Disease, Southeast University, Jiangsu, China).
Funding
This research was funded by the Science and Technology Innovation 2030 Major Projects (2022ZD0211600, CMX), the National Natural Science Foundation of China (82271574, 82071204, 81871069, CMX), the Foundation of Jiangsu Commission of Health (Z2018023, CMX), and Jiangsu Province Health Management Department of China (H2020080, HXF).
Author information
Authors and Affiliations
Consortia
Contributions
All authors have made substantial intellectual contributions to this manuscript. Dr. Xie took the conceptualization, analysis, and revision of this manuscript. Ms. Qi, Ms. He and Ms. Feng conducted the data collection and preparation. Ms. Wang and Ms. Qi conducted the analysis, investigation, visualization, and writing of this manuscript. All authors have given final approval of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. The funders had no role in the design of the study, or in the collection, analyses, or interpretation of data, or in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The data used in preparing this article were parted from the Depression Imaging REsearch ConsorTium.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Wang, Q., Qi, L., He, C. et al. Age- and gender-related dispersion of brain networks across the lifespan. GeroScience 46, 1303–1318 (2024). https://doi.org/10.1007/s11357-023-00900-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00900-8