Abstract
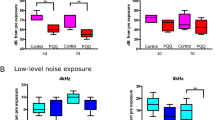
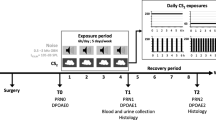
Hearing loss induced by noise and combinations of factors is a common occupational disease among workers. This study aimed to investigate the impact of acute exposure to white noise and Al2O3 NPs, alone and in combination, on changes in the hearing and structural functions of the cochlea in rats. Thirty-six rats were randomly assigned to one of six groups: Control, acute exposure to white noise, exposure to γ-Al2O3 NPs, exposure to noise plus γ-Al2O3 NPs, exposure to α-Al2O3 NPs, and exposure to the combination of noise plus α-Al2O3 NPs. TTS and PTS were examined using DPOAE, while oxidative index (MDA, GSH-Px), gene expression (NOX3, TGF-ß, CYP1A1), protein expression (ß-Tubulin, Myosin VII), and histopathological changes were examined in the cochlea. The morphology of Al2O3 NPs was examined by TEM. The results of the DPOAE test showed a significant increase in TTS in all groups and an increase in PTS in the groups exposed to noise, γ-Al2O3 NPs, and a combination of noise plus Al2O3 NPs (P < 0.05). In the group exposed to white noise plus Al2O3 NPs, the MDA levels increased, the level of GSH-Px decreased, and the expression percentage of ß-Tubulin and Myosin VII decreased, while the expression of NOX3, TGF-ß, and CYP1A1 (except for the α-Al2O3 NPs group) significantly increased (P < 0.05). Histopathological changes of the cochlea indicated damage to hair and ganglion cells, which was more severe in the combined exposure group. The combined and independent exposure to white noise and Al2O3 NPs damaged hair and ganglion cells for high-frequency perception, affecting the function and structure of the cochlea and leading to TTS and PTS.









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Al2O3-NPs:
-
Oxide Aluminum nanoparticles
- DPOAE:
-
Distortion product otoacoustic emissions
- PTS:
-
Permanent threshold shift
- TTS:
-
Temporary threshold shift
- MDA:
-
Malondialdehyde
- GSH-Px:
-
Glutathione peroxidase
- NADPH Oxidase NOX3:
-
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases 3
- TGF-β1:
-
Transforming growth factor β1
- CYP1A1:
-
Cytochrome P450 1A1
- γ:
-
Gamma
- α:
-
Alpha
- SPL:
-
Sound pressure level
- DAPI:
-
Blue-fluorescent 4, 6-diamidino-2-phenylindole
- SGNs:
-
Spiral ganglion cells
- HCs:
-
Hair cells
- IHCs:
-
Inner hair cells
- OHCs:
-
Outer hair cells
- SGCs:
-
Spiral ganglion cells
- NFs:
-
Nerve fibers
- TM:
-
Tectorial membrane
- NIHL:
-
Noise-induced hearing loss
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
References
Abouee-Mehrizi A, Rasoulzadeh Y, Kazemi T, Mehdipour A, Mesgari-Abbasi M (2022) Toxicopathological changes induced by combined exposure to noise and toluene in New Zealand White rabbits. Arh Hig Rada Toksikol 73:31–42
Adzemovic MZ, Zeitelhofer M, Leisser M, Köck U, Kury A, Olsson T (2016) Immunohistochemical analysis in the rat central nervous system and peripheral lymph node tissue sections. JoVE (Journal of Visualized Experiments), e50425
Aksoy F, Dogan R, Yenigun A, Veyseller B, Ozturan O, Ozturk B (2015) Thymoquinone treatment for inner-ear acoustic trauma in rats. J Laryngol Otol 129:38–45
Almeida JPM, Chen AL, Foster A, Drezek R (2011) In vivo biodistribution of nanoparticles. Nanomedicine 6:815–835
Ates M, Demir V, Arslan Z, Daniels J, Farah IO, Bogatu C (2015) Evaluation of alpha and gamma aluminum oxide nanoparticle accumulation, toxicity, and depuration in Artemia salina larvae. Environ Toxicol 30:109–118
Balasubramanyam A, Sailaja N, Mahboob M, Rahman M, Misra S, Hussain SM, Grover P (2009) Evaluation of genotoxic effects of oral exposure to aluminum oxide nanomaterials in rat bone marrow. Mutat Res 676:41–47
Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause K-H (2004) NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279:46065–46072
Braydich-Stolle LK, Speshock JL, Castle A, Smith M, Murdock RC, Hussain SM (2010) Nanosized aluminum altered immune function. ACS Nano 4:3661–3670
Brown DM, Brown AM, Willitsford AH, Dinello-Fass R, Airola MB, Siegrist KM, Thomas ME, Chang Y (2016) Lidar measurements of solid rocket propellant fire particle plumes. Appl Opt 55:4657–4669
Chen X-m, Ji S-f, Liu Y-h, Xue X-m, Xu J, Gu Z-h, Deng S-l, Liu C-d, Wang H, Chang Y-m (2020) Ginsenoside Rd ameliorates auditory cortex injury associated with military aviation noise-induced hearing loss by activating SIRT1/PGC-1α signaling pathway. Front Physiol 11:788
Chu PL, Wu CC, Hsu CJ, Wang YT, Wu KD (2007) Potential ototoxicity of aluminum in hemodialysis patients. Laryngoscope 117:137–141
Chun KJ, Lee CH, Kim KW, Lee SM, Kim SY (2021) Effects of Androgen Receptor Inhibition on Kanamycin-Induced Hearing Loss in Rats. Int J Mol Sci 22:5307
Clerc F, Pouyatos B (2022) A Case Study about Joining Databases for the Assessment of Exposures to Noise and Ototoxic Substances in Occupational Settings. Int J Environ Res Public Health 19:4455
Colombari GC, Rossato M, Feres O, Hyppolito MA (2011) Effects of hyperbaric oxygen treatment on auditory hair cells after acute noise damage. Eur Arch Otorhinolaryngol 268:49–56
Cook G (n.d.) Ototoxic drugs, chemicals and heavy metals in the workplace. Workplace Group: Preserving Human Capital at Work
Dereköy FS, Dündar Y, Aslan R, Cangal A (2001) Influence of noise exposure on antioxidant system and TEOAEs in rabbits. Eur Arch Otorhinolaryngol 258:518–522
Diao MF, Liu HY, Zhang YM, Gao WY (2003) Changes in antioxidant capacity of the guinea pig exposed to noise and the protective effect of alpha-lipoic acid against acoustic trauma. Sheng Li Xue Bao:[Acta Physiologica Sinica] 55:672–676
El-Hussainy E-HM, Hussein AM, Abdel-Aziz A, El-Mehasseb I (2016) Effects of aluminum oxide (Al2O3) nanoparticles on ECG, myocardial inflammatory cytokines, redox state, and connexin 43 and lipid profile in rats: possible cardioprotective effect of gallic acid. Can J Physiol Pharmacol 94:868–878
Farfalla AA, Beseler C, Achutan C, Rautiainen R (2022) Coexposure to solvents and noise as a risk factor for hearing loss in agricultural workers. J Occup Environ Med 64:754–760
Fechter LD, Chen G-D, Rao D, Larabee J (2000) Predicting exposure conditions that facilitate the potentiation of noise-induced hearing loss by carbon monoxide. Toxicol Sci 58:315–323
Fetoni AR, Ferraresi A, La Greca C, Rizzo D, Sergi B, Tringali G, Piacentini R, Troiani D (2008) Antioxidant protection against acoustic trauma by coadministration of idebenone and vitamin E. NeuroReport 19:277–281
Fetoni AR, Piacentini R, Fiorita A, Paludetti G, Troiani D (2009) Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res 1257:108–116
Fetoni AR, Mancuso C, Eramo SLM, Ralli M, Piacentini R, Barone E, Paludetti G, Troiani D (2010) In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience 169:1575–1588
Gedik Ö, Doğan R, Babademez MA, Karataş E, Aydın MŞ, Koçyiğit A, Eşrefoğlu M, Özturan O (2020) Therapeutic effects of metformin for noise induced hearing loss. Am J Otolaryngol 41:102328
Gong T-W, Fairfield DA, Fullarton L, Dolan DF, Altschuler RA, Kohrman DC, Lomax MI (2012) Induction of heat shock proteins by hyperthermia and noise overstimulation in Hsf1−/− mice. J Assoc Res Otolaryngol 13:29–37
Habybabady RH, Mortazavi SB, Khavanin A, Mirzaei R (2017) Effects of exposure to cigarette smoke on distortion-product otoacoustic emissions in rats. Health Scope 6
Habybabady RH, Mortazavi SB, Khavanin A, Mirzaei R, Arab MR, Mesbahzadeh B, Hoseini M, Mohammadi M (2018) Protective effects of n-acetyl-l-cysteine on the density of spiral ganglion cells and histological changes induced by continuous noise exposure in rats. Malay J Med Sci 25:48
Habybabady RH, Mohammadi M, Mortazavi SB, Khavanin A, Mirzaei R, Malvajerdi MS (2019) The effect of simultaneous exposure to cigarette smoke and noise on distortion product otoacoustic emissions in rats. Toxicol Ind Health 35:349–357
Han Y, Wang X, Chen J, Sha SH (2015) Noise-induced cochlear F-actin depolymerization is mediated via ROCK 2/p-ERM signaling. J Neurochem 133:617–628
Hu B (2012) Noise-induced structural damage to the cochlea, Noise-induced hearing loss. Springer, pp. 57–86
Hull MJ, Abraham JL (2002) Aluminum welding fume-induced pneumoconiosis. Hum Pathol 33:819–825
Hunter D, Milton R, Perry KM, Thompson D (1944) Effect of aluminium and alumina on the lung in grinders of duralumin aeroplane propellers. Br J Ind Med 1:159
Ivanov S, Zhuravsky S, Yukina G, Tomson V, Korolev D, Galagudza M (2012) In vivo toxicity of intravenously administered silica and silicon nanoparticles. Materials 5:1873–1889
Jacukowicz-Sobala I, Ociński D, Kociołek-Balawejder E (2015) Iron and aluminium oxides containing industrial wastes as adsorbents of heavy metals: application possibilities and limitations. Waste Manage Res 33:612–629
Jongkamonwiwat N, Ramirez MA, Edassery S, Wong AC, Yu J, Abbott T, Pak K, Ryan AF, Savas JN (2020) Noise exposures causing hearing loss generate proteotoxic stress and activate the proteostasis network. Cell Rep 33:108431
Juergens L, Bieniussa L, Voelker J, Hagen R, Rak K (2020) Spatio-temporal distribution of tubulin-binding cofactors and posttranslational modifications of tubulin in the cochlea of mice. Histochem Cell Biol 154:671–681
Karami E, Goodarzi Z, Chahardoli R, Ghazi Khansari M, Kiani M, Shahtaheri SJ (2022) Investigating the protective effects of aqueous extract of the wormwood plant (Artemisia absinthium) on alumina nanoparticle-induced pulmonary toxicity in male Wistar rats. J Health Safety Work 12:288–308
Kim HJ, Kang KY, Baek JG, Jo HC, Kim H (2006) Expression of TGFβ family in the developing internal ear of rat embryos. J Korean Med Sci 21:136–142
Kirkegaard M, Murai N, Risling M, Suneson A, Järlebark L, Ulfendahl M (2006) Differential gene expression in the rat cochlea after exposure to impulse noise. Neuroscience 142:425–435
Kopke RD, Jackson RL, Coleman JK, Liu J, Bielefeld EC, Balough BJ (2007) NAC for noise: from the bench top to the clinic. Hear Res 226:114–125
Lee CH, Lee D-h, Lee SM, Kim SY (2020) Otoprotective effects of zingerone on cisplatin-induced ototoxicity. Int J Mol Sci 21:3503
Li X, Zhang C, Zhang X, Wang S, Meng Q, Wu S, Yang H, Xia Y, Chen R (2015) An acetyl-L-carnitine switch on mitochondrial dysfunction and rescue in the metabolomics study on aluminum oxide nanoparticles. Part Fibre Toxicol 13:1–19
Liu Y, Chen Q, Xu Y (2020) Research progress in refractory sudden hearing loss: steroid therapy. J Int Med Res 48:0300060519889426
Lorito G, Giordano P, Prosser S, Martini A, Hatzopoulos S (2006) Noise-induced hearing loss: a study on the pharmacological protection in the Sprague Dawley rat with N-acetyl-cysteine. Acta Otorhinolaryngol Ital 26:133
Lu J, Li W, Du X, Ewert DL, West MB, Stewart C, Floyd RA, Kopke RD (2014) Antioxidants reduce cellular and functional changes induced by intense noise in the inner ear and cochlear nucleus. J Assoc Res Otolaryngol 15:353–372
Morsy GM, Abou El-Ala KS, Ali AA (2016a) Studies on fate and toxicity of nanoalumina in male albino rats: oxidative stress in the brain, liver and kidney. Toxicol Ind Health 32:200–214
Morsy GM, El-Ala KSA, Ali AA (2016b) Studies on fate and toxicity of nanoalumina in male albino rats: lethality, bioaccumulation and genotoxicity. Toxicol Ind Health 32:344–359
Moussavi-Najarkola S, Khavanin A, Mirzaei R, Salehnia M, Muhammadnejad A, Akbari M (2012) Noise-induced outer hair cells’ dysfunction and cochlear damage in rabbits. Iran Red Crescent Med J 14:647
Mukherjea D, Jajoo S, Sheehan K, Kaur T, Sheth S, Bunch J, Perro C, Rybak LP, Ramkumar V (2011) NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss. Antioxid Redox Signal 14:999–1010
Murillo-Cuesta S, Rodríguez-de la Rosa L, Contreras J, Celaya AM, Camarero G, Rivera T, Varela-Nieto I (2015) Transforming growth factor β1 inhibition protects from noise-induced hearing loss. Front Aging Neurosci 7:32
Naples JG, Miller LE, Ramsey A, Li D (2020) Cochlear protein biomarkers as potential sites for targeted inner ear drug delivery. Drug Deliv Transl Res 10:368–379
Nordmann AS, Bohne BA, Harding GW (2000) Histopathological differences between temporary and permanent threshold shift. Hear Res 139:13–30
Özdemir D, Özgür A, Kalkan Y, Terzi S, Tümkaya L, Yılmaz A, Çeliker M, Dursun E (2019) The protective effects of whortleberry extract against cisplatin-induced ototoxicity in rats. Braz J Otorhinolaryngol 85:55–62
Patlolla AK, Kumari SA, Madhusudhanachary P, Turner T, Tchounwou PB (2018) Biochemical and histopathological evaluation of Al2O3 nanomaterials in kidney of Wistar rats. Curr Top Biochem Res 19:1
Piriyawong V, Thongpool V, Asanithi P, Limsuwan P (2012) Preparation and characterization of alumina nanoparticles in deionized water using laser ablation technique. Journal of Nanomaterials 2012
Prabhakar P, Reddy UA, Singh S, Balasubramanyam A, Rahman M, Indu Kumari S, Agawane SB, Murty U, Grover P, Mahboob M (2012) Retracted: Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appl Toxicol 32:436–445
Prasad K (2019) Involvement of Oxidative Stress, Inflammation, and Glutamate in Ototoxici-ty, and their Attenuation by Simultaneous Activation of Nrf2 and Elevation of Antioxidant Compounds. Int J Neurol Neurother 6:83
Qian M, Wang Q, Wang Z, Ma Q, Wang X, Han K, Wu H, Huang Z (2021) Dose-dependent pattern of Cochlear synaptic degeneration in C57BL/6J mice induced by repeated noise exposure. Neural plasticity 2021
Rajiv S, Jerobin J, Saranya V, Nainawat M, Sharma A, Makwana P, Gayathri C, Bharath L, Singh M, Kumar M (2016) Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum Exp Toxicol 35:170–183
Rousset F, Nacher-Soler G, Kokje VBC, Sgroi S, Coelho M, Krause K-H, Senn P (2022) NADPH oxidase 3 deficiency protects from noise-induced sensorineural hearing loss. Frontiers in cell and developmental biology, 219
Satoh H, Billings P, Firestein GS, Harris JP, Keithley EM (2006) Transforming growth factor β expression during an inner ear immune response. Ann Otol Rhinol Laryngol 115:81–88
Sikkeland L, Alexis N, Fry R, Martin E, Danielsen TE, Søstrand P, Kongerud J (2016) Inflammation in induced sputum after aluminium oxide exposure: an experimental chamber study. Occup Environ Med 73:199–205
Sirko P, Kozlov AS (2022) Immunohistochemistry localises myosin-7a to cochlear efferent boutons. Wellcome Open Research 7
Sjostrand AP, Dogan R, Kocyigit A, Karatas E, Budak BB, Ozturan O (2016) Therapeutic efficacy of Ginkgo biloba for early-period noise-induced hearing loss: an experimental animal study. Am J Otolaryngol 37:416–424
Xing M, Zou H, Gao X, Chang B, Tang S, Zhang M (2015) Workplace exposure to airborne alumina nanoparticles associated with separation and packaging processes in a pilot factory. Environ Sci Process Impacts 17:656–666
Xiong H, Lai L, Ye Y, Zheng Y (2021) Glucose protects cochlear hair cells against oxidative stress and attenuates noise-induced hearing loss in mice. Neurosci Bull 37:657–668
Yamashita D, Jiang H-Y, Schacht J, Miller JM (2004) Delayed production of free radicals following noise exposure. Brain Res 1019:201–209
Yeo NK, Ahn YS, Kim JW, Choi SH, Lim GC, Chung JW (2011) Proteomic Analysis of the Protein Expression in the Cochlea of Noise-Exposed Mice. Korean J Audiol 15:107–113
Yu S, Liu F, Wang C, Zhang J, Zhu A, Zou L, Han A, Li J, Chang X, Sun Y (2018) Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol Med Rep 17:3133–3139
Yuan B-C, Su F-M, Wu W-T, Liu W-S, Chiu K-H (2012) A predictive model of the association between gene polymorphism and the risk of noise-induced hearing loss caused by gunfire noise. J Chin Med Assoc 75:36–39
Zahra G, Esmaeil K, Mohammad F, Rashidy-Pour A, Mahdi M, Mahdi A, Ali K (2022) Combined effects of the exposure to silver nanoparticles and noise on hearing function and cochlea structure of the male rats. Life Sci 304:120724
Zhu C, Cheng C, Wang Y, Muhammad W, Liu S, Zhu W, Shao B, Zhang Z, Yan X, He Q (2018) Loss of ARHGEF6 causes hair cell stereocilia deficits and hearing loss in mice. Front Mol Neurosci 11:362
Funding
This research was part of a Ph.D. thesis supported by Tehran University of Medical Sciences and Health Services (research grant number: 52889).
Author information
Authors and Affiliations
Contributions
EK was responsible for doing this project (related to his Ph.D. thesis); MK was responsible for histopathological analyses; ZG and MGK interpreted the data and cooperated in the revision of the manuscript; ARP and AK cooperated in the revision of the manuscript; SJS, designed the study and wrote and revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The research reported in this manuscript has been a registered project and received required permission for carrying out the project (related to Ph.D. thesis) from Institutional Ethical Review Board of Tehran University of Medical Sciences for this study. IR.TUMS.SPH.REC.1399.214.
Consent to publication
Not applicable.
Consent to participant
Not applicable.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the study, authorship, and/or publication of this paper.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahtaheri, S.J., Goodarzi, Z., Karami, E. et al. Effects of acute exposure to Al2O3-NPs (α and γ) and white noise and their combination on cochlea structure and function in Wistar rats. Environ Sci Pollut Res 30, 89859–89876 (2023). https://doi.org/10.1007/s11356-023-28745-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28745-w