Abstract
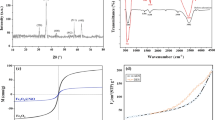
A novel fabrication of magnetite (Fe3O4) nanochains, surface functionalized with glutathione (GSH), has been attempted through a basic wet reduction method, coalesced with oxidative etching for the removal of crystal violet (CV) and phenol red (PR) from an aqueous solution. The structural and functional characterizations of GSH@Fe3O4 MNPs were performed using SEM-EDX, DLS, XRD, and FTIR. The nanochain-structured adsorbent was found to have an average size of 24 ± 1.29 nm and a zeta potential value of − 6.44 mV. The batch experiments showed that GSH@Fe3O4 MNPs have a brilliant removal efficiency of 97% and 79% for CV and PR dyes, respectively, within a period of 60 min. The influence of different operational parameters like adsorbent dosage, pH, temperature, reaction time, and initial dye concentration on the removal behaviour of the adsorbent was studied in detail. The adsorbate-adsorbent reaction was tested over isotherm models, and the reaction fitted well for Langmuir isotherm with an excellent qmax value of 1619.5 mg/g and 1316.16 mg/g for CV and PR dye, respectively. The experimental results were also validated using different reaction kinetics, and it was found that the pseudo-first-order model fits well for PR dye adsorption (R2 = 0.91), while adsorption of CV dye was in best agreement with the pseudo-second-order kinetic model (R2 = 0.98). Thermodynamic studies revealed that the adsorption reaction was spontaneous and endothermic in nature. Furthermore, GSH@Fe3O4 MNPs can be reused effectively up to 5 cycles of dye removal. Major mechanisms involved in the adsorption reaction were expected to be electrostatic attraction, hydrogen bonding, and π-interactions. The efficiency of GSH@Fe3O4 MNPs in real water samples suggested that it has a high potential for dye removal from complex aqueous systems and could be used as an effective alternative for remediation of dyes contaminated water.










Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Abbasian M, Jaymand M, Niroomand P, Farnoudian-Habibi A, Karaj-Abad SG (2017) Grafting of aniline derivatives onto chitosan and their applications for removal of reactive dyes from industrial effluents. Int J Biol Macromol 95:393–403
AbdEl-Salam AH, Ewais HA, Basaleh AS (2017) Silver nanoparticlesimmobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions. A kinetic study. J Mol Liq 248:833–841
Adlnasab L, Shabanian M, Ezoddin M, Maghsodi A (2017) Amine rich functionalized mesoporous silica for the effective removal of alizarin yellow and phenol red dyes from waste waters based on response surface methodology. Mater Sci Eng B 226:188–198
Ahmad PR, Hasan I, Mittal A (2017) Adsorption of Cr (VI) and Cd (II) on chitosan grafted polyaniline-OMMT nanocomposite: isotherms, kinetics and thermodynamics studies. Desalination Water Treat 58:144–153
Ahsaine HA, Anfar Z, Zbair M, Ezahri M, El Alem N (2018) Adsorptive removal of methylene blue and crystal violet onto micro-mesoporous Zr3O/activated carbon composite: a joint experimental and statistical modeling considerations. Journal of Chemistry 2018:1–14
Ai L, Zhang C, Liao F, Wang Y, Li M, Meng L, Jiang J (2011) Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater 198:282–290
Ali A, Hira Zafar MZ, ul Haq, I., Phull, A. R., Ali, J. S., & Hussain, A. (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9:49
Ali I, Peng C, Khan ZM, Sultan M, Naz I (2018) Green synthesis of phytogenic magnetic nanoparticles and their applications in the adsorptive removal of crystal violet from aqueous solution. Arabian Journal for Science and Engineering 43(11):6245–6259
Alves de Lima RO, Bazo AP, Salvadori DMF, Rech CM, de Palma Oliveira D, de Aragão Umbuzeiro G (2007) Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat Res Genet Toxicol Environ Mutagen 626:53–60
An S, Liu X, Yang L, Zhang L (2015) Enhancement removal of crystal violet dye using magnetic calcium ferrite nanoparticle: study in single- and binary-solute systems. Chem Eng Res Des 94:726–735
Arora C, Kumar P, Soni S, Mittal J, Mittal A, Singh B (2020) Efficient removal of malachite green dye from aqueous solution using Curcuma caesia based activated carbon. Desalination Water Treat 195:341–352
Arora C, Sonia S, Bajpaib PK, Mittalc J, Mariyam A (2021) Dye removal from waste water using metal organic frameworks. Management of Contaminants of Emerging Concern (CEC) in Environment 375
Badhai P, Kashyap S, Behera SK (2020) Adsorption of phenol red onto GO-Fe3O4 hybrids in aqueous media. Environ Nanotechnol Monit Manag 13:100282
Basu S, Panigrahi S, Praharaj S, Ghosh SK, Pande S, Jana S, Pal T (2006) Dipole–dipole plasmon interactions in self-assembly of gold organosol induced by glutathione. New J Chem 30(9):1333–1339
Beato-López JJ, Domínguez M, Ramírez-del-Solar M, Litrán R (2020) Glutathione-magnetite nanoparticles: synthesis and physical characterization for application as MRI contrast agent. SN Appl Sci 2:1202
Bordbar AK, Rastegari AA, Amiri R, Ranjbakhsh E, Abbasi M, Khosropour AR (2014) Characterization of modified magnetite nanoparticles for albumin immobilization. Biotechnology Research International. Article ID 705068:1–6
Chaki SH, Malek TJ, Chaudhary MD, Tailor JP, Deshpande MP (2015) Magnetite Fe 3 O 4 nanoparticles synthesis by wet chemical reduction and their characterization. Adv Nat Sci Nanosci Nanotechnol 6:035009
Chandhru M, Kutti Rani S, Vasimalai N (2020) Reductive degradation of toxic six dyes in industrial wastewater using diaminobenzoic acid capped silver nanoparticles. J Environ Chem Eng 8:104225
Cheng Y, Zhou F, Li S, Chen Z (2016) Removal of mixed contaminants, crystal violet, and heavy metal ions by using immobilized stains as the functional biomaterial. RSC Advances 6(72):67858–67865
Cheriaa J, Khaireddine M, Rouabhia M, Bakhrouf A (2012) Removal of triphenylmethane dyes by bacterial consortium. Sci World J 2012:512454
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences, and uses (Vol. 2, p. 71). Weinheim: Wiley-vch
Cozzoli PD, Pellegrino T, Manna L (2006) Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem Soc Rev 35:1195–1208
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155
Daraei H, Mittal A (2017) Investigation of adsorption performance of activated carbon prepared from waste tire for the removal of methylene blue dye from wastewater. Desalination Water Treat 90:294–298
Dawood S, Sen TK (2012) Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res 46:1933–1946
Dehghan Abkenar S (2016) Application of Magnetic-modified Fe3O4 Nanoparticles for Removal of Crystal Violet from Aqueous Solution: Kinetic, Equilibrium and Thermodynamic Studies. Journal of Applied Chemical Research 10(1):65–74
de Jesús Ruíz-Baltazar Á, Reyes-López SY, Pérez R (2017) Magnetic structures synthesized by controlled oxidative etching: structural characterization and magnetic behavior. Results Phys 7:1828–1832
Di Guardo A, Terzaghi E, Raspa G, Borin S, Mapelli F, Chouaia B, Zanardini E, Morosini C, Colombo A, Fattore E, Davoli E, Armiraglio S, Sale VM, Anelli S, Nastasio P (2017) Differentiating current and past PCB and PCDD/F sources: the role of a large contaminated soil site in an industrialized city area. Environ Pollut 223:367–375
Du C, Song Y, Shi S, Jiang B, Yang J, Xiao S (2020) Preparation and characterization of a novel Fe3O4-graphene-biochar composite for crystal violet adsorption. Sci Total Environ 711:134662
Feng L, Shen Y, Wu T, Liu B, Zhang D, Tang Z (2020) Adsorption equilibrium isotherms and thermodynamic analysis of CH4, CO2, CO, N2 and H2 on NaY Zeolite. Adsorption 26(7):1101–1111
Galdames A, Ruiz-Rubio L, Orueta M, Sánchez-Arzalluz M, Vilas-Vilela JL (2020) Zero-valent iron nanoparticles for soil and groundwater remediation. International Journal of Environmental Research and Public Health 17(16):5817
Gautam A, Rawat S, Verma L, Singh J, Sikarwar S, Yadav BC, Kalamdhad AS (2018) Green synthesis of iron nanoparticle from extract of waste tea: An application for phenol red removal from aqueous solution. Environmental Nanotechnology Monitoring and Management 10:377–387
Gita S, Hussan A, Choudhury TG (2017) Impact of textile dyes waste on aquatic environments and its treatment. Environ Ecol 35(3C):2349–2353
Gomes CS, Piccin JS, Gutterres M (2016) Optimizing adsorption parameters in tannery-dye-containing effluent treatment with leather shaving waste. Process Saf Environ Protect 99:98–106
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Gupta VK, Agarwal S, Ahmad R, Mirza A, Mittal J (2020) Sequestration of toxic congo red dye from aqueous solution using ecofriendly guar gum/activated carbonnanocomposite. International Journal of Biological Macromolecules 158:1310–1318
Huang L, He M, Chen B, Hu B (2018) Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere 199:435–444
Jabbar KQ, Barzinjy AA, Hamad SM (2022) Iron oxide nanoparticles: preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environmental Nanotechnology, Monitoring & Management, p 100661
Jain R, Sharma P, Sikarwar S, Mittal J, Pathak D (2014) Adsorption kinetics and thermodynamics of hazardous dye Tropaeoline 000 unto Aeroxide Alu C (Nano alumina): a non-carbon adsorbent. Desalination Water Treat 52:7776–7783
JeyaRanchani AA, Parthasarathy V, Mahalakshmi S, Anbarasan R (2019) Removal of hazardous pollutants from wastewater: catalytic applications of Mg nanoparticle functionalized aminoclay. J Mol Liquids 296:112005
Kakavandi B, Jonidi A, Rezaei R, Nasseri S, Ameri A, Esrafily A (2013) Synthesis and properties of Fe3O4-activated carbon magnetic nanoparticles for removal of aniline from aqueous solution: equilibrium, kinetic and thermodynamic studies. Iran J Environ Health Sci Eng 10:19
Kataria N, Garg VK (2017) Removal of Congo red and Brilliant green dyes from aqueous solution using flower shaped ZnO nanoparticles. J Environ Chem Eng 5:5420–5428
Kerkez D, Bečelić-Tomin M, Gvoić V, Dalmacija B (2021) Metal nanoparticles in dye wastewater treatment - smart solution for clear water. Recent Patents Nanotechnol 15:270–294
Khuntia BK, Anwar MF, Alam T, Samim M, Kumari M, Arora I (2019) Synthesis and characterization of zero-valent iron nanoparticles, and the study of their effect against the degradation of DDT in soil and assessment of their toxicity against collembola and ostracods. ACS omega 4(20):18502–18509
Kovalenko MV, Scheele M, Talapin DV (2009) Colloidal nanocrystals with molecular metal chalcogenide surface ligands. Science 324:1417
Kumar V, Saharan P, Sharma AK, Umar A, Kaushal I, Mittal A, Al-Hadeethi Y, Rashad B (2020) Silver doped manganese oxide-carbon nanotube nanocomposite for enhanced dye-sequestration: isotherm studies and RSM modelling approach. Ceram Int 46:10309–10319
Kumari P, Shekhar, Parashara H (2018) β-cyclodextrin modified magnetite nanoparticles for efficient removal of eosin and phloxine dyes from aqueous solution. Materials Today: Proceedings 5:15473–15480
Lakkaboyana SK, Khantong S, Asmel NK, Obaidullah S, Kumar V, Kannan K, Venkateswarlu K, Yuzir A, Wan Yaacob WZ (2021) Indonesian Kaolin supported nZVI (IK-nZVI) used for the an efficient removal of Pb(II) from aqueous solutions: kinetics, thermodynamics and mechanism. J Environ Chem Eng 9:106483
Lakkaboyana SK, Soontarapa K, Asmel NK, Kumar V, Marella RK, Yuzir A, Wan Yaacob WZ (2021) Synthesis and characterization of Cu(OH)2-NWs-PVA-AC nano-composite and its use as an efficient adsorbent for removal of methylene blue. Sci Rep 11:5686
Lakkaboyana SK, Soontarapa K, Vinaykumar Marella RK, Kannan K (2021) Preparation of novel chitosan polymeric nanocomposite as an efficient material for the removal of Acid Blue 25 from aqueous environment. Int J Biol Macromol 168:760–768
Lee D, Park M-K, Lee I-S, Choi S-D (2019) Contamination characteristics of siloxanes in coastal sediment collected from industrialized bays in South Korea. Ecotoxicol Environ Saf 182:109457
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290
Li Y, Zhou S, Jia Z, Ge L, Mei L, Sui X, Wang X, Li B, Wang J, Wu S (2018) Influence of industrialization and environmental protection on environmental pollution: a case study of Taihu Lake, China. Int J Environ Res Public Health 15:2628
Li Z, Li L, Hu D, Gao C, Xiong J, Jiang H, Li W (2019) Efficient removal of heavy metal ions and organic dyes with cucurbit [8] uril-functionalized chitosan. J Colloid Interface Sci 539:400–413
Lim J, Yeap SP, Che HX, Low SC (2013) Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res Lett 8:381
Lingamdinne LP, Choi J-S, Choi Y-L, Chang Y-Y, Yang J-K, Karri RR, Koduru JR (2020) Process modeling and optimization of an iron oxide immobilized graphene oxide gadolinium nanocomposite for arsenic adsorption. J Mol Liq 299:112261
Lu X, Liu Q, Huo G, Liang G, Sun Q, Song X (2012) CTAB-mediated synthesis of iron–nickel alloy nanochains and their magnetic properties. Colloids Surf A Physicochem Eng Aspects 407:23–28
Malik LA, Bashir A, Manzoor T, Pandith AH (2019) Microwave-assisted synthesis of glutathione-coated hollow zinc oxide for the removal of heavy metal ions from aqueous systems. RSC Adv 9:15976–15985
Mani S, Bharagava R (2015) Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev Environ Contam Toxicol 237:71–104
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK (2021) Adsorption behaviour of Chrysoidine R dye on a metal/halide-free variant of ordered mesoporous carbon. Desalination and Water Treatment Science and Engineering 223:425–433
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK (2021) Fixed-bed adsorption of the dye Chrysoidine R on ordered mesoporous carbon. Desalination and Water Treatment 229:395–402
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Efficient batch and Fixed-Bed sequestration of a basic dye using a novel variant of ordered mesoporous carbon as adsorbent. Arab J Chem 14:103186
Masoudian N, Rajabi M, Ghaedi M (2019) Titanium oxide nanoparticlesloaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of congo red and phenol red dyes from wastewaters. Polyhedron 173:114105
Mella B, Barcellos BSdC, da Silva Costa DE, Gutterres M (2018) Treatment of leather dyeing wastewater with associated process of coagulation-flocculation/adsorption/ozonation. Ozone Sci Eng 40:133–140
Micheletti L, Coldibeli B, Salamanca-Neto CAR, Almeida LC, Sartori ER (2020) Assessment of the use of boron-doped diamond electrode for highly sensitive voltammetric determination of the azo-dye carmoisine E−122 in food and environmental matrices. Talanta 220:121417
Mittal J (2020) Permissible synthetic food dyes in India. Reson J Sci Educ 25:567–577
Mittal J (2021) Recent progress in the synthesis of layered double hydroxides and their application for the adsorptive removal of dyes: a review. J Environ Manag 295:113017
Mittal A, Mittal J (2015) Hen feather: A remarkable adsorbent for dye removal. Green chemistry for dyes removal from wastewater 409–457
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interface Sci 337(2):345–354
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J Colloid Interface Sci 343(2):463–473
Mittal J, Ahmad PR, Mittal A (2021a) Kahwa tea (Camellia sinensis) carbon — a novel and green low-cost adsorbent for the sequestration of titan yellow dye from its aqueous solutions. Desalination Water Treat 227:404–411
Mittal J, Mariyam A, Sakina F, Baker RT, Sharma AK, Mittal A (2021b) Batch and bulk adsorptive removal of anionic dye using metal/halide-free ordered mesoporous carbon as adsorbent. J Clean Prod 321:129060
Mittal J, Ahmad R, Ejaz MO, Mariyam A, Mittal A (2021c) A novel, eco-friendly bio-nanocomposite (Alg-Cst/Kal) for the adsorptive removal of crystal violet dye from its aqueous solutions. International Journal of Phytoremediation 1–12
Monrás JP, Díaz V, Bravo D, Montes RA, Chasteen TG, Osorio-Román IO, Vásquez CC, Pérez-Donoso JM (2012) Enhanced Glutathione content allows the in vivo synthesis of fluorescent CdTe nanoparticles by Escherichia coli. PLoS One 7:e48657
Mustafa S, Dilara B, Nargis K, Naeem A, Shahida P (2002) Surface properties of the mixed oxides of iron and silica. Colloids Surf A Physicochem Eng Aspects 205:273–282
Nasiri J, Motamedi E, Naghavi MR, Ghafoori M (2019) Removal of crystal violet from water using β-cyclodextrin functionalized biogenic zero-valent iron nanoadsorbents synthesized via aqueous root extracts of Ferula persica. J Hazard Mater 367:325–338
Palani G, Arputhalatha A, Kannan K, Lakkaboyana SK, Hanafiah MM, Kumar V, Marella RK (2021) Current trends in the application of nanomaterials for the removal of pollutants from industrial wastewater treatment—a review. Molecules 26(9):2799
Patel A, Soni S, Mittal J, Mittal A, Arora C (2021) Desalination and Water Treatment Sequestration of crystal violet from aqueous solution using ash of black turmeric rhizome. Desalination Water Treat 220:342
Patra S, Roy E, Madhuri R, Sharma PK (2016) Agar based bimetallic nanoparticles as high-performance renewable adsorbent for removal and degradation of cationic organic dyes. Journal of Industrial and Engineering Chemistry 33:226–238
Rajput S, Pittman CU Jr, Mohan D (2016) Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removalfrom water. J Colloid Interface Sci 468:334–346
Sabzevari M, Cree DE, Wilson LD (2018) Graphene oxide–chitosan composite material for treatment of a model dye effluent. ACS Omega 3:13045–13054
Sadat SA, Ghaedi AM, Panahimehr M, Baneshi MM, Vafaei A, Ansarizadeh M (2019) Rapid room-temperature synthesis of cadmium zeolitic imidazolate framework nanoparticles based on 1,1′-carbonyldiimidazole as ultra-high-efficiency adsorbent for ultrasound-assisted removal of malachite green dye. Appl Surf Sci 467–468:1204–1212
Saharan P, Kumar V, Mittal J, Sharma V, Sharma AK (2021) Efficient ultrasonic assisted adsorption of organic pollutants employing bimetallic-carbon nanocomposites. Sep Sci Technol 56:2895–2908
Sharma P, Holliger N, Pfromm PH, Liu B, Chikan V (2020) Size-controlled synthesis of iron and iron oxide nanoparticles by the rapid inductive heating method. ACS omega 5(31):19853–19860
Sims RA, Harmer SL, Quinton JS (2019) The role of physisorption and chemisorption in the oscillatory adsorption of organosilanes on aluminium oxide. Polymers (Basel) 11:410
Singamaneni S, Bliznyuk VN, Binek C, Tsymbal EY (2011) Magnetic nanoparticles: recent advances in synthesis, self-assembly and applications. J Mater Chem 21:16819–16845
Soni S, Bajpai PK, Bharti D, Mittal J, Arora C (2020) Removal of crystal violet from aqueous solution using iron based metal organic framework. Desalin Water Treat 205:386–399
Sun P, Hui C, Azim Khan R, Du J, Zhang Q, Zhao YH (2015) Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Scientific Reports 5:12638
Sun M, Ricker K, Osborne G, Marder ME, Schmitz R (2018) Evidence on the carcinogenicity of gentian violet. Office of Enviromental Health Hazard Assessment 11–14
Talapin DV (2008) LEGO Materials. ACS Nano 2:1097–1100
Teja AS, Koh P-Y (2009) Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Crystal Growth Characterizat Mater 55:22–45
Teotia M, Mittal A, Soni RK (2019) Light-mediated thermoset polymers. In Materials for Biomedical Engineering (pp. 57–103). Elsevier
Tkaczyk A, Mitrowska K, Posyniak A (2020) Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ 717:137222
Vallinayagam S, Rajendran K, Lakkaboyana SK, Soontarapa K, R RR, Sharma VK, Kumar V, Venkateswarlu K, Koduru JR (2021): Recent developments in magnetic nanoparticles and nano-composites for wastewater treatment. J Environ Chem Eng 9:106553
Wahab HS, Hussain AA (2016) Photocatalytic oxidation of phenol red onto nanocrystalline TiO2 particles. J Nanostruct Chem 6:261–274
Waranya Chatuphonprasert KJ (2021) Effect of phenol red on cell cultures. Isan J Pharm Sci 17:13–23
Weber WJ, Morris JC (1963): Kinetics of adsorption on carbon from solution
Welshons WV, Wolf MF, Murphy CS, Jordan VC (1988) Estrogenic activity of phenol red. Mol Cell Endocrinol 57:169–78
Xia X, Zhou Z, Wu S, Wang D, Zheng S, Wang G (2018) Adsorption removal of multiple dyes using biogenic selenium nanoparticles from an Escherichia coli strain overexpressed selenite reductase CsrF. Nanomaterials 8(4):234
Xiao D, Zhang C, Yuan D, He J, Wu J, Zhang K, Lin R, He H (2014) Magnetic solid-phase extraction based on Fe3O4 nanoparticle retrieval of chitosan for the determination of flavonoids in biological samples coupled with high performance liquid chromatography. RSC Adv 4:64843–64854
Zafar AM, Javed MA, Hassan AA, Mohamed MM (2021) Groundwater remediation using zero-valent iron nanoparticles (nZVI). Groundwater for Sustainable Development 15:100694
Zamri MFMA, Bahru R, Suja F, Shamsuddin AH, Pramanik SK, Fattah IMR (2021) Treatment strategies for enhancing the removal of endocrine-disrupting chemicals in water and wastewater systems. J Water Process Eng 41:102017
Zhang F, Wang C-C (2008) Fabrication of One-dimensional iron oxide/silica nanostructures with high magnetic sensitivity by dipole-directed self-assembly. J Phys Chem C 112:15151–15156
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: A review. J Mol Liquids 214:175–191
Zhang S, Hu R, Li H (2019) Glutathione modified Ag nanoparticles as efficient detector for pyrimethanil. Nanotechnology 30:115502
Zheng Y, Yang Z, Li Y, Ying JY (2008) From glutathione capping to a crosslinked, phytochelatin-like coating of quantum dots. Adv Mater 20:3410–3415
Zheng Y, Zeng J, Ruditskiy A, Liu M, Xia Y (2014) Oxidative etching and its role in manipulating the nucleation and growth of noble-metal nanocrystals. Chem Mater 26:22–33
Zhou R, Shen N, Zhao J, Su Y, Ren H (2018) Glutathione-coated Fe3O4 nanoparticles with enhanced Fenton-like activity at neutral pH for degrading 2,4-dichlorophenol. J Mater Chem A 6:1275–1283
Zhu Y, Zhang X, Zhu J, Zhao Q, Li Y, Li W, Fan C, Huang Q (2012) Cytotoxicity of phenol red in toxicity assays for carbon nanoparticles. Int J Mol Sci 13:12336–12348
Acknowledgements
We are immensely thankful to the Material Research Centre (MRC), MNIT, Jaipur, Rajasthan, for providing instrument facilities, inevitably required for the completion of this paper. The authors are deeply indebted to the Head, Department of Environmental Science, Central University of Rajasthan, for providing all necessary facilities for this work. Financial support from the University Grant Commission (UGC), New Delhi, India, in the form of Senior Research Fellowship (provided to MB) is duly acknowledged.
Author information
Authors and Affiliations
Contributions
MB: Experiment planning and designing, data analysis, result interpretation, writing. NK: Sample preparation, characterization, spectral analysis, result interpretation. KR: Sample characterization and result interpretation. RS: Conceptualization, data analysis, and interpretation, editing, supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Behera, M., Kumari, N., Raza, K. et al. Fabrication of glutathione functionalized self-assembled magnetite nanochains for effective removal of crystal violet and phenol red dye from aqueous matrix. Environ Sci Pollut Res 29, 72260–72278 (2022). https://doi.org/10.1007/s11356-022-19520-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19520-4