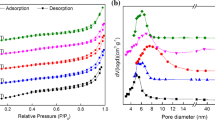
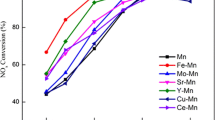
Abstract
A series of Ho-modified Mn/Ti catalysts with various content of Ho were prepared by impregnation method, and the low-temperature catalytic performance was tested. Techniques of BET, SEM, XRD, H2-TPR, and XPS were carried out to research the effects of Ho modification on the physicochemical properties of Mn/Ti catalysts. Results showed that appropriate Ho addition could reduce the starting temperature of Mn/TiO2 catalyst to 100 °C. 0.2HoMn/Ti exhibiting a wider temperature range of 140~220 °C with nearing 100% NOx conversion. It was found that the 0.2HoMn/TiO2 catalyst possessed a better dispersion of active component, enhanced redox capacity, a higher concentration of Mn4+ species, and a larger amount of Oβ content on the catalyst surface, which are all likely predominant factors related to the excellent SCR activity. Additionally, Ho improved the Lewis acid sites and enhanced the adsorption and activation ability of NH3, as well as the NO to NO2 oxidation ability. The selective catalytic reduction with ammonia (NH3-SCR) deNOx mechanism over HoMn/Ti catalysts obeyed both the Eley–Rideal (E-R) and Langmuir–Hinshelwood (L-H) mechanisms under low-temperature reaction conditions.












Similar content being viewed by others
References
Andonova S, Tamm S, Montreuil C, Lambert C, Olsson L (2016) The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl Catal B Environ 180:775–787
Cai HS, Liu GG, Lü W, Xiaoxia LI, Lin YU, Daguang LI (2008) Effect of Ho-doping on photocatalytic activity of nanosized TiO2 catalyst. J Rare Earth 26:71–75
Chen T, Guan B, He L, Lin Z (2014) In situ DRIFTS study of the mechanism of low temperature selective catalytic reduction over manganese-iron oxides. Chin J Catal 35:294–301
Chen G, Shi JW, Fan ZY, Yu YK, Niu CM (2017) Eu-Mn-Ti mixed oxides for the SCR of NOx with NH3: the effects of Eu-modification on catalytic performance and mechanism. Fuel Process Technol 167:322–333
Eng J, Bartholomew CH (1997) Kinetic and mechanistic study of NOx reduction by NH3 over H-form zeolites. J Catal 171:27–44
Ettireddy PR, Ettireddy N, Mamedov S, Boolchand P, Smirniotis PG (2007) Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl Catal B Environ 76:123–134
Guo RT, Wang SX, Pan WG, Li MY, Sun P, Liu SM, Sun X, Liu SW, Liu J (2017) Different poisoning effects of K and Mg on the Mn/TiO2 catalyst for selective catalytic reduction of NOx with NH3: a mechanistic study. J Phys Chem C 121:7881–7891
Gutiérrez-Alejandre A, Ramírez J, Busca G (1998) A vibrational and spectroscopic study of WO3/TiO2-Al2O3 catalyst precursors. Langmuir 14:630–639
Hadjiivanov K, Knözinger H (2000) Species formed after NO adsorption and NO + O2 co-adsorption on TiO2: an FTIR spectroscopic study. Phys Chem Phys 2:2803–2806
Hang H, Cai SX, Li HR, Lei H, Shi LY, Zhang DS (2016) Mechanistic aspects of deNOx processing over TiO2 supported co-Mn oxide catalysts: structure-activity relationships and in situ DRIFTs analysis. ACS Catal 5:6069–6077
Hua SB, Tian HZ, Wang K, Zhu CY, Gao JJ, Ma YL, Xue YF, Yong W, Duan SH, Zhou JR (2016) Atmospheric emission inventory of hazardous air pollutants from China’s cement plants: temporal trends, spatial variation characteristics and scenario projections. Atmos Environ 128:1–9
Jiang HX, Wang CX, Wang HQ, Zhang MH (2016) Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater Lett 168:17–19
Kijlstra WS, Brands DS, Smit HI, Poels EK, Bliek A (1997) Mechanism of the selective catalytic reduction of NO with NH3 over MnOx/Al2O3. J Catal 171:219–230
Kim J, Shin BK, Yooh SH, Lee SH, Lim HM, Jeong YK (2012) Effects of manganese precursors on MnOx/TiO2 for low-temperature SCR of NOx. J Korean Met Mater 50:201–205
Larrubia MA, Ramis G, Busca G (2001) An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3-TiO2 SCR catalysts. Appl Catal B Environ 30:101–110
Li GB, Zhu BZ, Sun YL, Yin SL, Zi ZH, Fang QL, Ge TT, Li JX (2018a) Study of the alkali metal poisoning resistance of a Co-modified Mn/Ni foam catalyst in low-temperature flue gas SCR DeNOx. J Mater Sci 53:9674–9689
Li W, Zhang C, Li X, Tan P, Zhou AL, Fang QY, Chen G (2018b) Ho-modified Mn-Ce/TiO2 for low-temperature SCR of NOx with NH3: evaluation and characterization. Chin J Catal 39:1653–1663
Lietti L, Ramis G, Berti F, Toledo G, Robba D, Busca G, Forzatti P (1998) Chemical, structural and mechanistic aspects on NOx SCR over commercial and model oxide catalysts. Catal Today 42:101–116
Liu Y, You XC, Sheng ZY, Ma DR, Yu DQ, Xiang X, Sheng W (2018) The promoting effect of noble metal (Rh, Ru, Pt, Pd) doping on the performances of MnOx-CeO2/graphene catalysts for the selective catalytic reduction of NO with NH3 at low temperatures. New J Chem 42:11673–11681
Meng DM, Zhan WC, Guo Y, Guo YL, Wang YS, Wang L, Lu GZ (2016) A highly effective catalyst of Sm-Mn mixed oxide for the selective catalytic reduction of NOx with ammonia: effect of the calcination temperature. J Mol Catal A Chem 420:272–281
Mu JC, Li XY, Sun WB, Fan SY, Zhang DK (2018) Enhancement of low-temperature catalytic activity over a highly dispersed Fe-Mn/Ti catalyst for selective catalytic reduction of NOx with NH3. Ind Eng Chem Res 57:10159–10169
Peng S, Guo RT, Liu SM, Wang SX, Pan WG, Li MY (2016) The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl Catal A Gen 531:129–138
Ramis G, Yi L, Busca G (1996) Ammonia activation over catalysts for the selective catalytic reduction of NOx and the selective catalytic oxidation of NH3. An FT-IR study. Catal Today 28:373–380
Shi JW, Zheng JT, Hu Y, Zhao YC (2007) Influence of Fe3+ and Ho3+ co-doping on the photocatalytic activity of TiO2. Mater Chem 106:247–249
Simas AM, Freire RO, Rocha GB (2008) Lanthanide coordination compounds modeling: sparkle/PM3 parameters for dysprosium (III), holmium (III) and erbium (III). J Organomet Chem 693:1952–1956
Thirupathi B, Smirniotis PG (2011) Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl Catal B Environ 110:195–206
Thirupathi B, Smirniotis PG (2012) Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: catalytic evaluation and characterizations. J Catal 288:74–83
Wang XM, Li XY, Zhao QD, Sun WB, Tade M, Liu SM, (2016) Improved activity of W-modified MnO -TiO , catalysts for the selective catalytic reduction of NO with NH. Chem Eng J 288:216–222
Wu ZB, Jiang BQ, Liu Y, Wang HQ, Jin RB (2007) DRIFT study of manganese/titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Environ Sci Technol 41:5812–5817
Wu ZB, Tang NA, Xiao L, Liu Y, Wang HC (2010) MnOx/TiO2 composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation. J Colloid Interf Sci 352:143–148
Wu SG, Yao XJ, Zhang L, Cao Y, Zou WX, Li L, Ma K, Tang CJ, Gao F, Dong L (2015a) Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem Commun 51:3470–3473
Wu SG, Zhang L, Wang XB, Zou WX, Cao Y, Sun JF, Tang CJ, Gao F, Deng Y, Dong L (2015b) Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl Catal A Gen 505:235–242
Wu SG, Zhang L, Wang XB, Zou WX, Cao Y, Sun JF, Tang CJ, Gao F Deng Y, Dong L, (2015c) Synthesis, characterization and catalytic performance of FeMnTiO mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH -SCR. Appl Catal A-Gen 505:235–242
Wu ZB, Jiang BQ, Liu Y, (2008) Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl Catal B-Environ 79:347–355
Yang SJ, Qi FH, Yong L, Xiong SC, Yi L, Fu YW, Shan WP, Li JH (2014) Dual effect of sulfation on the selective catalytic reduction of NO with NH3 over MnOx/TiO2: key factor of NH3 distribution. Ind Eng Chem Res 53:5810–5819
Yu CL, Huang BC, Dong LF, Feng C, Liu XQ (2016) In situ FT-IR study of highly dispersed MnOx/SAPO-34 catalyst for low-temperature selective catalytic reduction of NOx by NH3. Catal Today 281:610–620
Zhang YP, Wang XL, Shen K, Xu HT, Sun KQ, Zhou CC (2012) WO3 modification of MnOx/TiO2 catalysts for low temperature selective catalytic reduction of NO with ammonia. Chin J Catal 33:1523–1531
Zhang L, Zhang DS, Zhang JP, Cai SX, Fang C, Huang L, Li HR, Gao RH, Shi L (2013) Design of meso-TiO2@MnOx-CeOx/CNTs with a core-shell structure as DeNOx catalysts: promotion of activity, stability and SO2-tolerance. Nanoscale 5:9821–9829
Zhang WK, Chen HF, Yang X, Fang RY, Liu XS (2018) Effects of Nd-modification on the activity and SO2 resistance of MnOx/TiO2 catalyst for low-temperature NH3-SCR. New J Chem 42:12845–12852
Zhu YW, Zhang YP, Xiao R, Huang TJ, Shen K, (2017) Novel holmium-modified Fe-Mn/TiO catalysts with a broad temperature window and high sulfur dioxide tolerance for low-temperature SCR. Catal Commun 88:64–67
Zhuang K, Qiu J, Tang FS, Xu BL, Fan YN (n.d.) The structure and catalytic activity of anatase and rutile titania supported manganese oxide catalysts for selective catalytic reduction of NO by NH3. Phys Chem Chem Phys 13:4463–4469
Funding
This work was supported by the National Key R&D Program of China (2017YFB0603201), Key Research and Development Projects of Jiangsu Province (BE2017716), and Environmental nonprofit industry research subject (2016YFC0208102).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Santiago V. Luis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wu, P., Li, G. et al. Improved activity of Ho-modified Mn/Ti catalysts for the selective catalytic reduction of NO with NH3. Environ Sci Pollut Res 27, 26954–26964 (2020). https://doi.org/10.1007/s11356-020-07749-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07749-w