Abstract
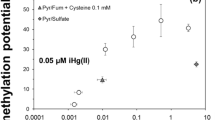
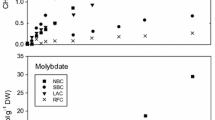
The speciation of mercury (Hg) is a major determinant of its methylation rate by sulfate-reducing bacteria (SRB), considered the primary methylators. Under anoxic conditions, sulfur (S) cycling may have a significant influence on Hg complexation and methylation, by influencing both SRB activity and the pool of available reduced S ligands, as the presence of zero-valent sulfur (S(0)) in sulfidic water results in the formation of polysulfides. While SRB frequently coexist with S-oxidizing bacteria in natural environments, the effect that these organisms may have on methylation by SRB is not understood. In this study, we investigate the role of S(0) in methylation by SRB monocultures and cocultures with phototrophic green or purple S-oxidizing bacteria. In the coculture experiments, the presence of S-oxidizers was found to increase Hg methylation rates, apparently by maintaining favorable chemical speciation in the environment. The measured Hg methylation rates were in accord with predictions based on geochemical modeling of speciation. In SRB monoculture experiments conducted in the presence and absence of S(0), the data showed that at limited total Hg, the presence of polysulfides resulted in decreased Hg methylation, presumably by causing a decrease in the most bioavailable Hg–sulfide complexes. These results indicate that models of Hg speciation and methylation in the environment should include a detailed investigation of S redox speciation.






Similar content being viewed by others
References
Acha, D., Iniguez, V., Roulet, M., Guimaraes, J., Luna, R., Alanoca, L., & Sanchez, S. (2005). Sulfate-reducing bacteria in floating macrophyte rhizospheres from an Amazonian floodplain lake in Bolivia and their association with Hg methylation. Applied and Environmental Microbiology, 71(11), 7531–7535.
Barkay, T., Gillman, M., & Turner, R. R. (1997). Effects of dissolved organic carbon and salinity on bioavailability of mercury. Applied and Environmental Microbiology, 63(11), 4267–4271.
Barkay, T., Kritee, K., Boyd, E., & Geesey, G. (2010). A thermophilic bacterial origin and subsequent constraints by redox, light and salinity on the evolution of the microbial mercuric reductase. Environmental Microbiology, 12(11), 2904–2917.
Barkay, T., Miller, S. M., & Summers, A. O. (2003). Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiology Reviews, 27(2–3), 355–384.
Benoit, J. M., Gilmour, C. C., Heyes, A., Mason, R. P., & Miller, C. (2003). Geochemical and biological controls over methylmercury production and degradation in aquatic systems. In Y. Cai & O. C. Braids (Eds.), Biogeochemistry of environmentally important trace elements; ACS Symposium Series 835 (pp. 262–297). Washington, DC: American Chemical Society.
Benoit, J. M., Gilmour, C. C., & Mason, R. P. (2001). The Influence of sulfide on solid-phase mercury availability for methylation by pure cultures of Desulfobulbus propionicus (1pr3). Environmental Science & Technology, 35(1), 127–132.
Benoit, J. M., Gilmour, C. C., Mason, R. P., Riedel, G. S., & Riedel, G. F. (1998). Behavior of mercury in the Patuxent River estuary. Biogeochemistry, 40(2–3), 249–265.
Benoit, J., Gilmour, C., Mason, R., & Heyes, A. (1999). Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environmental Science & Technology, 33(6), 951–957.
Benoit, J. M., Mason, R. P., & Gilmour, C. C. (1999). Estimation of mercury-sulfide speciation in sediment pore waters using octanol-water partitioning and implications for availability to methylating bacteria. Environmental Toxicology and Chemistry, 18(10), 2138–2141.
Biebl, H., & Pfennig, N. (1977). Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Archives of Microbiology, 112(1), 115–117.
Borchardt, L. G., & Easty, D. B. (1984). Gas chromatographic determination of elemental and polysulfide sulfur in kraft pulping liquors. Journal of Chromatography A, 299, 471–476.
Boulegue, J., Lord, C., & Church, T. (1982). Sulfur speciation and associated trace metals (Fe, Cu) in the pore waters of Great Marsh, Delaware. Geochimica et Cosmochimica Acta, 46(3), 453–464.
Branfireun, B., Bishop, K., Roulet, N., Granberg, G., & Nilsson, M. (2001). Mercury cycling in boreal ecosystems: the long-term effect of acid rain constituents on peatland pore water methylmercury concentrations. Geophysical Research Letters, 28(7), 1227–1230.
Burmolle, M., Webb, J., Rao, D., Hansen, L., Sorensen, S., & Kjelleberg, S. (2006). Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Applied and Environmental Microbiology, 72(6), 3916–3923.
Cleckner, L., Gilmour, C., Hurley, J., & Krabbenhoft, D. (1999). Mercury methylation in periphyton of the Florida Everglades. Limnology and Oceanography, 44(7), 1815–1825.
Clesceri, L. S., Greenberg, A. E., & Eaton, A. D. (Eds.). (1999). Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association.
Compeau, G., & Bartha, R. (1985). Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Applied and Environmental Microbiology, 50(2), 498–502.
Craig, P., & Moreton, P. (1983). Total mercury, methyl mercury, and sulphide in River Carron sediments. Marine Pollution Bulletin, 14(11), 408–411.
Daskalakis, K. D., & Helz, G. R. (1993). The solubility of sphalerite (ZnS) in sulfidic solutions at 25 °C and 1 atm pressure. Geochimica et Cosmochimica Acta, 57(20), 4923–4931.
Desrosiers, M., Planas, D., & Mucci, A. (2006). Mercury methylation in the epilithon of boreal shield aquatic ecosystems. Environmental Science & Technology, 40(5), 1540–1546.
Dyrssen, D. (1989). Biogenic sulfur in two different marine environments. Marine Chemistry, 28(1–3), 241–249.
Dyrssen, D., & Wedborg, M. (1991). The sulfur-mercury(II) system in natural waters. Water Air & Soil Pollution, 56(1), 507–519.
Fitzgerald, W., Engstrom, D., Mason, R., & Nater, E. (1998). The case for atmospheric contamination in remote areas. Environmental Science & Technology, 32(1), 1–7.
Fleming, E., Mack, E., Green, P., & Nelson, D. (2006). Mercury methylation from unexpected sources: molybdate-inhibited freshwater sediments and an iron-reducing bacterium. Applied and Environmental Microbiology, 72(1), 457–464.
Gilmour, C. C., Elias, D. A., Kucken, A. M., Brown, S. D., Palumbo, A. V., Schadt, C. W., & Wall, J. D. (2011). Sulfate-reducing bacterium Desulfovibrio desulfuricans ND132 as a model for understanding bacterial mercury methylation. Applied and Environmental Microbiology, 77(12), 3938–3951.
Gilmour, C., Henry, E., & Mitchell, R. (1992). Sulfate stimulation of mercury methylation in fresh-water sediments. Environmental Science & Technology, 26(11), 2281–2287.
Gilmour, C. C., Riedel, G. F., Ederington, M. C., Bell, J. T., Benoit, J. M., Gill, G. A., & Stordal, M. C. (1998). Methylmercury concentrations and production rates across a trophic gradient in the northern Everglades. Biogeochemistry, 40(2–3), 327–345.
Golding, G., Kelly, C., Sparling, R., Loewen, P., Rudd, J., & Barkay, T. (2002). Evidence for facilitated uptake of Hg(II) by Vibrio anguillarum and Escherichia coli under anaerobic and aerobic conditions. Limnology and Oceanography, 47(4), 967–975.
Goulet, R., Holmes, J., Page, B., Poissant, L., Siciliano, S., Lean, D., Wang, F., Amyot, M., & Tessier, A. (2007). Mercury transformations and fluxes in sediments of a riverine wetland. Geochimica et Cosmochimica Acta, 71(14), 3393–3406.
Gubeli, A., & Ste-Marie, J. (1967). Constantes de stabilite de thiocomplexes et produits de solubilite de sulfures de metaux: II. Sulfure de zinc Canadian Journal of Chemistry, 45, 2101–2108.
Hamelin, S., Amyot, M., Barkay, T., Wang, Y., & Planas, D. (2011). Methanogens: principal methylators of mercury in lake periphyton. Environmental Science & Technology, 45(18), 7693–7700.
Henry, E. (1992). The role of sulfate-reducing bacteria in environmental mercury methylation. Ph.D. thesis, Harvard University.
Hsu-Kim, H., & Sedlak, D. (2005). Similarities between inorganic sulfide and the strong Hg(II)-complexing ligands in municipal wastewater effluent. Environmental Science & Technology, 39(11), 4035–4041.
Ito, T., Okabe, S., Satoh, H., & Watanabe, Y. (2002). Successional development of sulfate-reducing bacterial populations and their activities in a wastewater biofilm growing under microaerophilic conditions. Applied and Environmental Microbiology, 68(3), 1392–1402.
Jay, J.A. (1999). Effect of polysulfides on cinnabar solubility, partitioning, and methylation by Desulfovibrio desulfuricans. Ph.D. Dissertation, Massachusetts Institute of Technology.
Jay, J. A., Morel, F. M. M., & Hemond, H. F. (2000). Mercury speciation in the presence of polysulfides. Environmental Science & Technology, 34(11), 2196–2200.
Jay, J. A., Murray, K. J., Gilmour, C. C., Mason, R. P., Morel, F. M. M., Roberts, A. L., & Hemond, H. F. (2002). Mercury methylation by Desulfovibrio desulfuricans ND132 in the presence of polysulfides. Applied and Environmental Microbiology, 68(11), 5741–5745.
Jorgensen, B. B. (1983). The microbial sulphur cycle. In M. Geochemistry (Ed.), Krumbein. W.E: Blackwell Scientific Publications.
Kelly, C. A., Rudd, J. W. M., & Holoka, M. H. (2003). Effect of pH on mercury uptake by an aquatic bacterium: implications for Hg cycling. Environmental Science & Technology, 37(13), 2941–2946.
Kerin, E., Gilmour, C., Roden, E., Suzuki, M., Coates, J., & Mason, R. (2006). Mercury methylation by dissimilatory iron-reducing bacteria. Applied and Environmental Microbiology, 72(12), 7919–7921.
King, S., Behnke, S., Slack, K., Krabbenhoft, D., Nordstrom, D., Burr, M., & Striegl, R. (2006). Mercury in water and biomass of microbial communities in hot springs of Yellowstone National Park, USA. Applied Geochemistry, 21(11), 1868–1879.
King, J., Saunders, F., Lee, R., & Jahnke, R. (1999). Coupling mercury methylation rates to sulfate reduction rates in marine sediments. Environmental Toxicology and Chemistry, 18(7), 1362–1369.
Kusel, K., Trinkwalter, T., Drake, H. L., & Devereux, R. (2006). Comparative evaluation of anaerobic bacterial communities associated with roots of submerged macrophytes growing in marine or brackish water sediments. Journal of Experimental Marine Biology and Ecology, 337(1), 49–58.
Lehnherr, I., & St Louis, V. (2009). Importance of ultraviolet radiation in the photodemethylation of methylmercury in freshwater ecosystems. Environmental Science & Technology, 43(15), 5692–5698.
Lin, C.-C., & Jay, J. A. (2007). Mercury methylation by planktonic and biofilm cultures of Desulfovibrio desulfuricans. Environmental Science & Technology, 41(19), 6691–6697.
Luther, G. W., Church, T., Scudlark, J., & Cosman, M. (1986). Inorganic and organic sulfur cycling in salt-marsh pore waters. Science, 232(4751), 746–749.
Luther, G. W., Glazer, B., Hohmann, L., Popp, J., Taillefert, M., Rozan, T., Brendel, P., Theberge, S., & Nuzzio, D. (2001). Sulfur speciation monitored in situ with solid state gold amalgam voltammetric microelectrodes: polysulfides as a special case in sediments, microbial mats and hydrothermal vent waters. Journal of Environmental Monitoring, 3(1), 61–66.
MacCrehan, W., & Shea, D. (1995). Temporal relationship of thiols to inorganic sulfur compounds in anoxic Chesapeake Bay sediment porewater. In M. A. Vairavamurthy, M. A. A. Schoonen, T. I. Eglinton, G. W. Luther, & B. Manowitz (Eds.), Geochemical transformations of sedimentary sulfur (ACS Symposium Series 612th ed., pp. 294–310). Washington, D C: American Chemical Society.
Marvin-DePasquale, M. C., & Oremland, R. S. (1998). Bacterial methylmercury degradation in Florida Everglades peat sediment. Environmental Science & Technology, 32(17), 2556–2563.
Mason, R. P., Fitzgerald, W. F., A K Hanson, J., Donaghay, P. L., & Sieburth, J. M. (1993). Mercury biogeochemical cycling in a stratified estuary. Limnology and Oceanography, 38(6), 1227–1241.
Mason, R., Reinfelder, J., & Morel, F. (1995). Bioaccumulation of mercury and methylmercury. Water Air & Soil Pollution, 80(1–4), 915–921.
Mason, R., Reinfelder, J., & Morel, F. (1996). Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environmental Science & Technology, 30(6), 1835–1845.
Masse, A., Pringault, O., & de Wit, R. (2002). Experimental study of interactions between purple and green sulfur bacteria in sandy sediments exposed to illumination deprived of near-infrared wavelengths. Applied and Environmental Microbiology, 68(6), 2972–2981.
Mauro, J., Guimaraes, J., Hintelman, H., Watras, C., Haack, E., & Coelho-Souza, S. (2002). Mercury methylation in macrophytes, periphyton, and water– comparative studies with stable and radio-mercury additions. Analytical and Bioanalytical Chemistry, 374(6), 983–989.
Miller, P., Vasudevan, D., Gschwend, P., & Roberts, A. (1998). Transformation of hexachloroethane in a sulfidic natural water. Environmental Science & Technology, 32(9), 1269–1275.
Mitchell, C. P. J., & Gilmour, C. C. (2008). Methylmercury production in a Chesapeake Bay salt marsh. Journal of Geophysical Research. doi:10.1029/2008JG000765.
Moench, Y., & Zeikus, J. (1983). An improved preparation method for a titanium(III) media reductant. Journal of Microbiological Methods, 1(4), 199–202.
Morel, F., Kraepiel, A., & Amyot, M. (1998). The chemical cycle and bioaccumulation of mercury. Annual Review of Ecology and Systematics, 29, 543–566.
Najera, I., Lin, C.-C., & Kohbodi, G. A. (2005). Effect of chemical speciation on the toxicity of mercury to Escherichia coli biofilms and planktonic cultures. Environmental Science & Technology, 39(9), 3116–3120.
Oremland, R. S., Culbertson, C. W., & Winfrey, M. R. (1991). Methylmercury decomposition in sediments and bacterial cultures: involvement of methanogens and sulfate reducers in oxidative demethylation. Applied and Environmental Microbiology, 57(1), 130–137.
Pak, K.-R., & Bartha, R. (1998). Mercury methylation by interspecies hydrogen and acetate transfer between sulfidogens and methanogens. Applied and Environmental Microbiology, 64(6), 1987–1990.
Palmer, R., Kazmerzak, K., Hansen, M., & Kolenbrander, P. (2001). Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infection and Immunity, 69(9), 5794–5804.
Paquette, K. (1994). Solubility of cinnabar (red HgS) and implications for mercury speciation in sulfidic waters. Ph.D. Dissertation, University of Maryland.
Paquette, K. E., & Helz, G. R. (1997). Inorganic speciation of mercury in sulfidic waters: the importance of zero-valent sulfur. Environmental Science & Technology, 31(7), 2148–2153.
Parker, J., & Bloom, N. (2005). Preservation and storage techniques for low-level aqueous mercury speciation. Science of the Total Environment, 337(1–3), 253–263.
Pathmanathan, S., Cardona-Castro, N., Sanchez-Jimenez, M., Correa-Ochoa, M., Puthucheary, S., & Thong, K. (2003). Simple and rapid detection of Salmonella strains by direct PCR amplification of the hilA gene. Journal of Medical Microbiology, 52(9), 773–776.
Pawlowicz, R., Baldwin, S. A., Muttray, A., Schmidtova, J., Laval, B., & Lamont, G. (2007). Physical, chemical, and microbial regimes in an anoxic fjord (Nitinat Lake). Limnology and Oceanography, 52(3), 1002–1017.
Prange, A., Engelhardt, H., Truper, H. G., & Dahl, C. (2004). The role of the sulfur globule proteins of Allochromatium vinosum: mutagenesis of the sulfur globule protein genes and expression studies by real-time RT-PCR. Archives of Microbiology, 182(2–3), 165–174.
Ravenschlag, K., Sahm, K., & Amann, R. (2001). Quantitative molecular analysis of the microbial community in marine Arctic sediments (Svalbard). Applied and Environmental Microbiology, 67(1), 387–395.
Rozan, T. F., Theberge, S., & Luther, G. (2000). Quantifying elemental sulfur (S0), bisulfide (HS-) and polysulfides (S x 2−) using a voltammetric method. Analytica Chimica Acta, 415, 175–184.
Schaefer, J., & Morel, F. (2009). High methylation rates of mercury bound to cysteine by Geobacter sulfurreducens. Nature Geoscience, 2(2), 123–126.
Schaefer, J. K., Rocks, S. S., Zheng, W., Liang, L., Gu, B., & Morel, F. M. M. (2011). Active transport, substrate specificity, and methylation of Hg(II) in anaerobic bacteria. Proceedings of the National Academy of Sciences of the USA, 108(21), 8714–8719.
Skyllberg, U. (2008). Competition among thiols and inorganic sulfides and polysulfides for Hg and MeHg in wetland soils and sediments under suboxic conditions: illumination of controversies and implications for MeHg net production. Journal of Geophysical Research. doi:10.1029/2008JG000745.
Skyllberg, U., Xia, K., Bloom, P., Nater, E., & Bloom, W. (2000). Binding of mercury(II) to reduced sulfur in soil organic matter along upland-peat soil transects. Journal of Environmental Quality, 29(3), 855–865.
Slowey, A. J. (2010). Rate of formation and dissolution of mercury sulfide nanoparticles: the dual role of natural organic matter. Geochimica et Cosmochimica Acta, 74(16), 4693–4708.
Stein, W., & Lieb, W. (1986). Transport and diffusion across cell membranes. Inc: Academic.
Ste-Marie, J., Torma, A., & Gubeli, A. (1964). The stability of thiocomplexes and solubility products of metal sulphides: I. Cadmium sulfide. Canadian Journal of Chemistry, 42(3), 662–668.
Steudel, R. (2003). Inorganic polysulfides S n 2− and radical anions S n − (review). Topics in Current Chemistry, 231, 127–152.
Taylor, B. F., A, H. T., & Pope, L. A. (1989). Analysis of sulfur as TPPS by high-performance liquid chromatography: applications to studies of sulfur bioproduction and sulfur in marine sediments. Journal of Microbiological Methods, 9(3), 221–231.
Then, J., & Truper, H. (1983). Sulfide oxidation in Ectothiorhodospira abdelmalekii. Evidence for the catalytic role of cytochrome c-551. Archives of Microbiology, 135(4), 254–258.
USEPA (2001). Method 1630: methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. EPA-821-R-01-020. Washington, DC: US Environmental Protection Agency: Office of Water Regulations and Standards, Criteria and Standards Division.
USEPA (2002). Method 1631: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. EPA-821-r-02-019. Washington, DC: US Environmental Protection Agency: Office of Water Regulations and Standards, Criteria and Standards Division.
vanGemerden, H. (1993). Microbial mats: a joint venture. Marine Geology, 113(1–2), 3–25.
vanGemerden, H. (1967). On the bacterial sulphur cycle of inland waters. Ph.D. Thesis, Universidad of Leiden
Visscher, P. T., Nijburg, J. W., & Gemerden, H. (1990). Polysulfide utilization by Thiocapsa roseopersicina. Archives of Microbiology, 155(1), 75–81.
Visscher, P. T., & vanGemerden, H. (1993). Sulfur cycling in laminated marine microbial ecosystems. In R. Oremland (Ed.), Biogeochemistry of global change. New York: Chapman & Hall.
Weber, J. H. (1993). Review of possible paths for abiotic methylation of mercury(II) in the aquatic environment. Chemosphere, 26(11), 2063–2077.
Webster, N. S., & Negri, A. P. (2006). Site-specific variation in Antarctic marine biofilms established on artificial surfaces. Environmental Microbiology, 8(7), 1177–1190.
Weng, F., & Tessier, A. (2009). Zero-valent sulfur and metal speciation in sediment porewaters of freshwater lakes. Environmental Science & Technology, 43(19), 7252–7257.
Widdel, F., & Bak, F. (1992). The prokaryotes. A handbook on the biology of bacteria. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, & K. H. Sceleifer (Eds.), Ecophysiology, isolation, identification, and applications (pp. 3352–3378). New York: Spring-Verlag.
Winfrey, M. R., & Rudd, J. W. M. (1990). Environmental-factors affecting the formation of methylmercury in low pH lakes. Environmental Toxicology and Chemistry, 9(7), 853–869.
Zhang, T., & Fang, H. H. P. (2001). Phylogenetic diversity of a SRB-rich marine biofilm. Applied Microbiology and Biotechnology, 57(3), 437–440.
Zhang, T., & Hsu-Kim, H. (2010). Photolytic degradation of methylmercury enhanced by binding to natural organic ligands. Nature Geoscience, 3(7), 473–476.
Acknowledgments
This work was supported by a CAREER grant (BES-0348783) from the National Science Foundation to Jenny A. Jay. We thank all of our lab colleagues at UCLA for their constant support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kampalath, R.A., Lin, CC. & Jay, J.A. Influences of Zero-Valent Sulfur on Mercury Methylation in Bacterial Cocultures. Water Air Soil Pollut 224, 1399 (2013). https://doi.org/10.1007/s11270-012-1399-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-012-1399-7