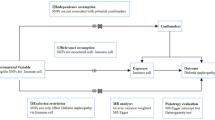
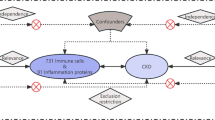
Abstract
Background
Diabetic nephropathy (DN) is one of the diabetic microvascular complications with complex pathophysiology, and exploring the landscape of immune dysregulation in DN is valuable for pathogenesis and disease treatment. We crystallized possible inflammatory exposures into 91 circulating inflammatory proteins and 109 blood immune cells; and assessed the causal relationship between inflammation and DN using Mendelian randomization (MR).
Methods
Based on publicly available genetic data, we explored causal associations between inflammation and DN risk by two-sample MR analysis. Genome-wide association study (GWAS) summary statistics for 91 circulating inflammatory proteins, 109 immune cells absolute counts, and DN were acquired from the GWAS Catalog. Inverse Variance Weighted (IVW) was the main MR method, while MR-Egger and MR-pleiotropy residuals and outliers (MR-PRESSO) were utilized for sensitivity analysis. Cochrane’s Q was used to test for heterogeneity. The leave-one-out method ensured the stability of the MR results.
Results
This study revealed that higher levels of TNF-related activation-induced cytokine and tumor necrosis factor ligand superfamily member 14 were possibly associated with the increased risk of DN according to the IVW approach, with estimated odds ratios (OR) of 1.287 (95% confidence interval [CI] 1.051 to 1.577, P = 0.015) and 1.249 (95% CI 1.018 to 1.532, P = 0.033). Five immune cell traits were identified that might be linked to increased DN risk, including the higher absolute counts of HLA DR+ natural killer cell (OR = 1.248, 95% CI 1.055 to 1.476, P = 0.010), IgD+ CD38+ B cell (OR = 1.148, 95% CI 1.033 to 1.276, P = 0.010), CD25++ CD8+ T cell (OR = 1.159, 95% CI 1.032 to 1.302, P = 0.013), CD4− CD8− T cell (OR = 1.226, 95% CI 1.032 to 1.457, P = 0.020), and IgD− CD38− B cell (OR = 1.182, 95% CI 1.009 to 1.386, P = 0.039). In addition, elevated levels of interleukin-1 alpha (OR = 0.712, 95% CI 0.514 to 0.984, P = 0.040) and unswitched memory B cell (OR = 0.797, 95% CI 0.651 to 0.974, P = 0.027) may reduce the risk of developing DN.
Conclusion
We identified inflammation-related exposures that may be associated with the risk of DN at the level of genetic prediction, which contributes to a better understanding of the etiologic of DN and facilitates the development of targeted therapies for DN.






Similar content being viewed by others
Data availability
The GWAS summary data used for these analyses are all publicly available in the online EBI GWAS Catalog.
References
Wu Y, Xiong T, Tan X, Chen L (2022) Frailty and risk of microvascular complications in patients with type 2 diabetes: a population-based cohort study. BMC Med 20:473. https://doi.org/10.1186/s12916-022-02675-9
Zelnick LR, Weiss NS, Kestenbaum BR et al (2017) Diabetes and CKD in the United States Population, 2009–2014. Clin J Am Soc Nephrol 12:1984–1990. https://doi.org/10.2215/CJN.03700417
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395:709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L et al (2020) Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci 21:3798. https://doi.org/10.3390/ijms21113798
Li Y, Wang L, Zhang J et al (2023) Integrated multi-omics and bioinformatic methods to reveal the mechanisms of sinomenine against diabetic nephropathy. BMC Complement Med Ther 23:287. https://doi.org/10.1186/s12906-023-04119-0
Mohanraj N, Prasanth S, Rajapriya P et al (2023) Bisphenol a accelerates the vascular complications in patients with Type 2 diabetes mellitus through vascular calcification-a molecular approach. Int Arch Occup Environ Health 96:1291–1299. https://doi.org/10.1007/s00420-023-02007-1
AlTamimi JZ, AlFaris NA, Alshammari GM et al (2023) Protective effect of eriodictyol against hyperglycemia-induced diabetic nephropathy in rats entails antioxidant and anti-inflammatory effects mediated by activating Nrf2. Saudi Pharm J 31:101817. https://doi.org/10.1016/j.jsps.2023.101817
Feigerlová E, Battaglia-Hsu S-F (2017) IL-6 signaling in diabetic nephropathy: from pathophysiology to therapeutic perspectives. Cytokine Growth Factor Rev 37:57–65. https://doi.org/10.1016/j.cytogfr.2017.03.003
Zhu H-M, Liu N, Sun D-X, Luo L (2023) Machine-learning algorithm-based prediction of a diagnostic model based on oxidative stress-related genes involved in immune infiltration in diabetic nephropathy patients. Front Immunol 14:1202298. https://doi.org/10.3389/fimmu.2023.1202298
Jin J, Wang L, Liu Y et al (2022) Depiction of immune heterogeneity of peripheral blood from patients with type II diabetic nephropathy based on mass cytometry. Front Endocrinol (Lausanne) 13:1018608. https://doi.org/10.3389/fendo.2022.1018608
Barutta F, Bruno G, Grimaldi S, Gruden G (2015) Inflammation in diabetic nephropathy: moving toward clinical biomarkers and targets for treatment. Endocrine 48:730–742. https://doi.org/10.1007/s12020-014-0437-1
Wang X, Gao Y, Yi W et al (2021) Inhibition of miRNA-155 alleviates high glucose-induced podocyte inflammation by targeting SIRT1 in diabetic mice. J Diabetes Res 2021:5597394. https://doi.org/10.1155/2021/5597394
Feng B, Lu Y, Ye L et al (2022) Mendelian randomization study supports the causal association between serum cystatin C and risk of diabetic nephropathy. Front Endocrinol (Lausanne) 13:1043174. https://doi.org/10.3389/fendo.2022.1043174
Sakaue S, Kanai M, Tanigawa Y et al (2021) A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet 53:1415–1424. https://doi.org/10.1038/s41588-021-00931-x
Zhao JH, Stacey D, Eriksson N et al (2023) Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol 24:1540–1551. https://doi.org/10.1038/s41590-023-01588-w
Orrù V, Steri M, Sidore C et al (2020) Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet 52:1036–1045. https://doi.org/10.1038/s41588-020-0684-4
Yeung CHC, Schooling CM (2021) Systemic inflammatory regulators and risk of Alzheimer’s disease: a bidirectional Mendelian-randomization study. Int J Epidemiol 50:829–840. https://doi.org/10.1093/ije/dyaa241
Xiang M, Wang Y, Gao Z et al (2022) Exploring causal correlations between inflammatory cytokines and systemic lupus erythematosus: a Mendelian randomization. Front Immunol 13:985729. https://doi.org/10.3389/fimmu.2022.985729
Palmer TM, Lawlor DA, Harbord RM et al (2012) Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Method Med Res 21:223–242. https://doi.org/10.1177/0962280210394459
Verbanck M, Chen C-Y, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:693–698. https://doi.org/10.1038/s41588-018-0099-7
Sproviero W, Winchester L, Newby D et al (2021) High blood pressure and risk of dementia: a two-sample Mendelian randomization study in the UK Biobank. Biol Psychiatry 89:817–824. https://doi.org/10.1016/j.biopsych.2020.12.015
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32:377–389. https://doi.org/10.1007/s10654-017-0255-x
Min J-K, Kim Y-M, Kim SW et al (2005) TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol 175:531–540. https://doi.org/10.4049/jimmunol.175.1.531
Chen X-W, Du X-Y, Wang Y-X et al (2016) Irbesartan ameliorates diabetic nephropathy by suppressing the RANKL-RANK-NF-κB pathway in type 2 diabetic db/db mice. Mediators Inflamm 2016:1405924. https://doi.org/10.1155/2016/1405924
Ke G, Chen X, Liao R et al (2021) Receptor activator of NF-κB mediates podocyte injury in diabetic nephropathy. Kidney Int 100:377–390. https://doi.org/10.1016/j.kint.2021.04.036
Mauri DN, Ebner R, Montgomery RI et al (1998) LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8:21–30. https://doi.org/10.1016/s1074-7613(00)80455-0
Halvorsen B, Santilli F, Scholz H et al (2016) LIGHT/TNFSF14 is increased in patients with type 2 diabetes mellitus and promotes islet cell dysfunction and endothelial cell inflammation in vitro. Diabetologia 59:2134–2144. https://doi.org/10.1007/s00125-016-4036-y
Li Y, Tang M, Han B et al (2020) Tumor necrosis factor superfamily 14 is critical for the development of renal fibrosis. Aging (Albany NY) 12:25469–25486. https://doi.org/10.18632/aging.104151
Qiu Y, Tang J, Zhao Q et al (2023) From diabetic nephropathy to end-stage renal disease: the effect of chemokines on the immune system. J Diabetes Res 2023:3931043. https://doi.org/10.1155/2023/3931043
Sandholm N, Forsblom C, Mäkinen V-P et al (2014) Genome-wide association study of urinary albumin excretion rate in patients with type 1 diabetes. Diabetologia 57:1143–1153. https://doi.org/10.1007/s00125-014-3202-3
Sanz I, Wei C, Lee FE-H, Anolik J (2008) Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol 20:67–82. https://doi.org/10.1016/j.smim.2007.12.006
Wangriatisak K, Kochayoo P, Thawornpan P et al (2022) CD4+ T-cell cooperation promoted pathogenic function of activated naïve B cells of patients with SLE. Lupus Sci Med 9:e000739. https://doi.org/10.1136/lupus-2022-000739
Frasca D, Diaz A, Romero M, Blomberg BB (2017) Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol 87:113–120. https://doi.org/10.1016/j.exger.2016.12.001
Wu S, Li W, Chen B et al (2022) Gene-based network analysis reveals prognostic biomarkers implicated in diabetic tubulointerstitial injury. Dis Markers 2022:2700392. https://doi.org/10.1155/2022/2700392
Wu Z, Zheng Y, Sheng J et al (2022) CD3+CD4-CD8-(Double-negative) T cells in inflammation immune Disorders and Cancer. Front Immunol 13:816005. https://doi.org/10.3389/fimmu.2022.816005
Crispín JC, Oukka M, Bayliss G et al (2008) Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 181:8761–8766. https://doi.org/10.4049/jimmunol.181.12.8761
Lisowska KA, Storoniak H, Dębska-Ślizień A (2022) T cell subpopulations and cytokine levels in hemodialysis patients. Hum Immunol 83:134–143. https://doi.org/10.1016/j.humimm.2021.11.003
Galozzi P, Bindoli S, Doria A, Sfriso P (2021) The revisited role of interleukin-1 alpha and beta in autoimmune and inflammatory disorders and in comorbidities. Autoimmun Rev 20:102785. https://doi.org/10.1016/j.autrev.2021.102785
Cavalli G, Colafrancesco S, Emmi G et al (2021) Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev 20:102763. https://doi.org/10.1016/j.autrev.2021.102763
Salti T, Khazim K, Haddad R et al (2020) glucose induces IL-1α-dependent inflammation and extracellular matrix proteins expression and deposition in renal tubular epithelial cells in diabetic kidney disease. Front Immunol 11:1270. https://doi.org/10.3389/fimmu.2020.01270
Acknowledgements
We would like to sincerely appreciate all GWAS participants and researchers for publicly sharing the summary statistics.
Author information
Authors and Affiliations
Contributions
HZ and YR conceived the study. YR accessed and acquired the data, performed the data analysis, prepared tables and figures, and wrote the manuscript. YR and HZ revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, Y., Zhang, H. The causal effect of inflammatory proteins and immune cell populations on diabetic nephropathy: evidence from Mendelian randomization. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04017-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04017-5