Abstract
Background
Diabetic nephropathy (DN) is a frequent diabetes complication with complex pathogenesis. Circular RNA (circRNA) circTAOK1 (also named circ_0003928) has been reported to be upregulated in high glucose (HG)-treated human umbilical vein endothelial cells. Also, exosomal circRNAs can exert significant roles in the pathology of various diseases. This study is designed to explore the role and mechanism of exosomal circTAOK1 on the glomerular mesangial cell (GMC) injury in DN.
Methods
Exosomes were detected by a transmission electron microscope. The protein levels of CD9, CD63, proliferating cell nuclear antigen (PCNA), cyclinD1, α-SMA, fibronectin, E-cadherin, N-cadherin, and SMAD family member 3 (SMAD3) were examined by western blot assay. circTAOK1, microRNA-520h (miR-520h), and SMAD3 levels were detected by real-time quantitative polymerase chain reaction (RT-qPCR). Cell proliferation and cell cycle progression were detected by cell counting kit-8 (CCK-8), 5-ethynyl-2′-deoxyuridine (EdU), and flow cytometry assays. The binding relationship between miR-520h and circTAOK1 or SMAD3 was predicted by Starbase and then verified by a dual-luciferase reporter and RNA immunoprecipitation (RIP), RNA pull-down assays.
Results
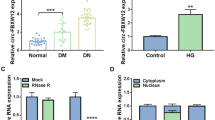
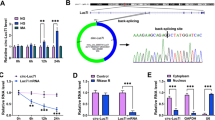
CircTAOK1 expression was upregulated in the exosomes isolated from HG-treated glomerular epithelial cells (GEC). Moreover, GEC-circTAOK1-Exo could promote proliferation, fibrosis, and epithelial–mesenchymal transition (EMT) of glomerular mesangial cells (GMCs). Mechanically, circTAOK1 could regulate SMAD3 expression by sponging miR-520h, GEO-si-circTAOK1 Exo-induced miR-520h and repressed SMAD3 expression in GMC.
Conclusion
GEC-circTAOK1-Exo could boost proliferation, fibrosis, and EMT of GMC through targeting the miR-520h/SMAD3 axis, providing new insights into the pathogenesis of DN.







Similar content being viewed by others
References
Flyvbjerg A (2017) The role of the complement system in diabetic nephropathy. Nat Rev Nephrol 13(5):311–318. https://doi.org/10.1038/nrneph.2017.31
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895. https://doi.org/10.1053/j.ajkd.2017.10.026
Tziomalos K, Athyros VG (2015) Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud 12(1–2):110–118. https://doi.org/10.1900/rds.2015.12.110
Meza Letelier CE, San Martín Ojeda CA, Ruiz Provoste JJ, Frugone Zaror CJ (2017) Pathophysiology of diabetic nephropathy: a literature review. Medwave 17(1):e6839. https://doi.org/10.5867/medwave.2017.01.6839
Yoon JJ, Lee HK, Kim HY, Han BH, Lee HS, Lee YJ et al (2020) Sauchinone protects renal mesangial cell dysfunction against angiotensin ii by improving renal fibrosis and inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms21197003
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28(1):164–176. https://doi.org/10.2337/diacare.28.1.164
Hombach S, Kretz M (2016) Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol 937:3–17. https://doi.org/10.1007/978-3-319-42059-2_1
Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH et al (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447(7146):799–816. https://doi.org/10.1038/nature05874
Lasda E, Parker R (2014) Circular RNAs: diversity of form and function. RNA 20(12):1829–1842. https://doi.org/10.1261/rna.047126.114
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J (2019) The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 20(11):675–691. https://doi.org/10.1038/s41576-019-0158-7
Zhang Z, Yang T, Xiao J (2018) Circular RNAs: promising biomarkers for human diseases. EBioMedicine 34:267–274. https://doi.org/10.1016/j.ebiom.2018.07.036
Zaiou M (2020) circRNAs signature as potential diagnostic and prognostic biomarker for diabetes mellitus and related cardiovascular complications. Cells. https://doi.org/10.3390/cells9030659
Loganathan TS, Sulaiman SA, Abdul Murad NA, Shah SA, Abdul Gafor AH, Jamal R et al (2020) Interactions among non-coding RNAs in diabetic nephropathy. Front Pharmacol 11:191. https://doi.org/10.3389/fphar.2020.00191
Zhang JR, Sun HJ (2020) Roles of circular RNAs in diabetic complications: from molecular mechanisms to therapeutic potential. Gene 763:145066. https://doi.org/10.1016/j.gene.2020.145066
Wang Q, Cang Z, Shen L, Peng W, Xi L, Jiang X et al (2021) circ_0037128/miR-17-3p/AKT3 axis promotes the development of diabetic nephropathy. Gene 765:145076. https://doi.org/10.1016/j.gene.2020.145076
Chen B, Li Y, Liu Y, Xu Z (2019) circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol 234(11):21249–21259. https://doi.org/10.1002/jcp.28730 (PMID: 31087368)
Jin G, Wang Q, Hu X, Li X, Pei X, Xu E et al (2019) Profiling and functional analysis of differentially expressed circular RNAs in high glucose-induced human umbilical vein endothelial cells. FEBS Open Bio 9(9):1640–1651. https://doi.org/10.1002/2211-5463.12709
An L, Ji D, Hu W, Wang J, Jin X, Qu Y et al (2020) Interference of Hsa_circ_0003928 alleviates high glucose-induced cell apoptosis and inflammation in HK-2 cells via miR-151-3p/Anxa2. Diabetes Metab Syndr Obes 13:3157–3168. https://doi.org/10.2147/dmso.s265543
Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902
Bang C, Thum T (2012) Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 44(11):2060–2064. https://doi.org/10.1016/j.biocel.2012.08.007 (PMID: 22903023)
Simons M, Raposo G (2009) Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21(4):575–581. https://doi.org/10.1016/j.ceb.2009.03.007
Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W et al (2019) Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer 18(1):116. https://doi.org/10.1186/s12943-019-1041-z
Zhou S, Fang J, Hu M, Pan S, Liu D, Xing G et al (2021) Determining the influence of high glucose on exosomal lncRNAs, mRNAs, circRNAs and miRNAs derived from human renal tubular epithelial cells. Aging (Albany NY) 13(6):8467–8480. https://doi.org/10.18632/aging.202656
Ling L, Tan Z, Zhang C, Gui S, Cui Y, Hu Y et al (2019) CircRNAs in exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells. Am J Transl Res 11(8):4667–4682
Thomas LF, Sætrom P (2014) Circular RNAs are depleted of polymorphisms at microRNA binding sites. Bioinformatics 30(16):2243–2246. https://doi.org/10.1093/bioinformatics/btu257
Qi H, Yao L, Liu Q (2020) NORAD affects the progression of diabetic nephropathy through targeting miR-520h to upregulate TLR4. Biochem Biophys Res Commun 521(1):190–195. https://doi.org/10.1016/j.bbrc.2019.10.102
Loh CY, Chai JY, Tang TF, Wong WF, Sethi G, Shanmugam MK et al (2019) The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. https://doi.org/10.3390/cells8101118
Mathieu M, Martin-Jaular L, Lavieu G, Théry C (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21(1):9–17. https://doi.org/10.1038/s41556-018-0250-9
Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C (2015) Exosome-based strategies for diagnosis and therapy. Recent Pat CNS Drug Discov 10(1):10–27. https://doi.org/10.2174/1574889810666150702124059
Mao R, Shen J, Hu X (2021) BMSCs-derived exosomal microRNA-let-7a plays a protective role in diabetic nephropathy via inhibition of USP22 expression. Life Sci 268:118937. https://doi.org/10.1016/j.lfs.2020.118937
Hao Y, Miao J, Liu W, Cai K, Huang X, Peng L (2021) Mesenchymal stem cell-derived exosomes carry microRNA-125a to protect against diabetic nephropathy by targeting histone deacetylase 1 and downregulating endothelin-1. Diabetes Metab Syndr Obes. 14:1405–1418. https://doi.org/10.2147/dmso.s286191
Su H, Qiao J, Hu J, Li Y, Lin J, Yu Q et al (2020) Podocyte-derived extracellular vesicles mediate renal proximal tubule cells dedifferentiation via microRNA-221 in diabetic nephropathy. Mol Cell Endocrinol 518:111034. https://doi.org/10.1016/j.mce.2020.111034
Fanale D, Taverna S, Russo A, Bazan V (2018) Circular RNA in exosomes. Adv Exp Med Biol 1087:109–117. https://doi.org/10.1007/978-981-13-1426-1_9
Jin J, Sun H, Shi C, Yang H, Wu Y, Li W et al (2020) Circular RNA in renal diseases. J Cell Mol Med 24(12):6523–6533. https://doi.org/10.1111/jcmm.15295
Zhou X, Hurst RD, Templeton D, Whiteside CI (1995) High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest 73(3):372–383
Chen SJ, Lv LL, Liu BC, Tang RN (2020) Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif 53(3):e12763. https://doi.org/10.1111/cpr.12763
Tung CW, Hsu YC, Shih YH, Chang PJ, Lin CL (2018) Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton) 23(Suppl 4):32–37. https://doi.org/10.1111/nep.13451
Rao N, Wang X, Xie J, Li J, Zhai Y, Li X et al (2019) Stem cells from human exfoliated deciduous teeth ameliorate diabetic nephropathy in vivo and in vitro by inhibiting advanced glycation end product-activated epithelial-mesenchymal transition. Stem Cells Int. 2019:2751475. https://doi.org/10.1155/2019/2751475
Wolf G (2000) Cell cycle regulation in diabetic nephropathy. Kidney Int Suppl 77:S59-66. https://doi.org/10.1046/j.1523-1755.2000.07710.x
Lei D, Chengcheng L, Xuan Q, Yibing C, Lei W, Hao Y et al (2019) Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol Res 146:104320. https://doi.org/10.1016/j.phrs.2019.104320
Chen S, Jim B, Ziyadeh FN (2003) Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 23(6):532–543. https://doi.org/10.1053/s0270-9295(03)00132-3
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388. https://doi.org/10.1038/nature11993
Panda AC (2018) Circular RNAs act as miRNA sponges. Adv Exp Med Biol 1087:67–79. https://doi.org/10.1007/978-981-13-1426-1_6
Zhu QJ, Zhu M, Xu XX, Meng XM, Wu YG (2019) Exosomes from high glucose-treated macrophages activate glomerular mesangial cells via TGF-β1/Smad3 pathway in vivo and in vitro. Faseb j 33(8):9279–9290. https://doi.org/10.1096/fj.201802427RRR
Liu X, Elia AE, Law SF, Golemis EA, Farley J, Wang T (2000) A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. Embo J 19(24):6759–6769. https://doi.org/10.1093/emboj/19.24.6759
Xu BH, Sheng J, You YK, Huang XR, Ma RCW, Wang Q et al (2020) Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism 103:154013. https://doi.org/10.1016/j.metabol.2019.154013
Funding
This work was supported by Science and Technology Talent Cultivation Project of Tianjin Municipal Health Commission (KJ20053).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2022_3139_MOESM1_ESM.tif
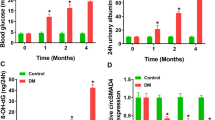
Supplementary file1 Figure S1 Exosome-mediated crcTAOK1 expression is up-regulated in the serum of DN patients. (A) The representative micrograph of round-shaped vesicles using electron microscopy. (B) Western blot for the protein levels of CD9 and CD63 in the exosomes of DN patients. (C) The expression level of exosomal crcTAOK1 using RT-qPCR in serum exosomes from 15 healthy volunteers and 20 DN patients. (D) ROC curve analysis for the diagnostic value evaluation of exosomal crcTAOK1 in DN patients. ***P <0.001. (TIF 618 KB)
Rights and permissions
About this article
Cite this article
Li, B., Sun, G., Yu, H. et al. Exosomal circTAOK1 contributes to diabetic kidney disease progression through regulating SMAD3 expression by sponging miR-520h. Int Urol Nephrol 54, 2343–2354 (2022). https://doi.org/10.1007/s11255-022-03139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03139-y