Abstract
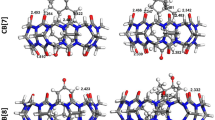
Binding affinity and intermolecular interactions are essential characteristics that could be used to comprehend molecular recognition between molecules in supramolecular host-guest systems. This work presented a molecular docking simulation and density functional theory (DFT) calculation at the B3LYP-631g(d) level of theory on dillapiole and its derivatives (guest compounds) complexation with cucurbit[7]uril (host compound). The supramolecular host-guest inclusion complex binding energies, − 4.46 to − 5.47 kcal mol−1 and − 0.53 to − 15.38 kcal mol−1 for docking and DFT calculation, respectively, were calculated, and the intermolecular interactions such as the hydrogen bonding, electrostatic, dispersion, and pi-alkyl formation involved were observed. The negative binding energies of D1-CB [7], D2-CB [7], D3-CB [7], D4-CB [7], D5-CB [7], D6-CB [7], and D-CB [7], computed from both the theoretical approaches, suggested the possible inclusion of the guests inside the cucurbit[7]uril cavity, enabling the formation of stable inclusion compounds. However, the significant difference in the binding energy values from the DFT calculation demonstrated a clustered preference in terms of the complex stabilisation, with D2-CB [7], D3-CB [7], D5-CB [7], and D6-CB [7] dominantly favourable, while D1-CB [7], D4-CB [7], and D were inclusion complexes with the least favourable. Encapsulation of the guests’ structural frame as a whole or a part and steric constraint associated with the guests’ substituents positioning in addition to the intermolecular interactions were also noted to induce stabilisation in the binding energy, thus reflecting a preferable inclusion complex. Besides, the theoretical calculations on the rationalisation of the selected guests’ energy barrier were found to correlate well with experimental works of hydroboration oxidation synthesis to produce alcohol derivatives of dillapiole.







Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Schneider H-J, Agrawal P, Yatsimirsky AK (2013) Supramolecular complexations of natural products. Chem Soc Rev 42:6777
Webber MJ, Langer R (2017) Drug delivery by supramolecular design. Chem Soc Rev 46:6600–6620
Takashima Y, Harada A (2017) Functioning via host–guest interactions. J Incl Phenom Macrocycl Chem 87:313–330
Tang J-H, Li Y, Wu Q et al (2019) Single-molecule level control of host-guest interactions in metallocycle-C60 complexes. Nat Commun 10:4599
Ma X, Zhao Y (2015) Biomedical applications of supramolecular systems based on host–guest interactions. Chem Rev 115:7794–7839
Al-Dubaili N, El-Tarabily K, Saleh N (2018) Host-guest complexes of imazalil with cucurbit[8]uril and β-cyclodextrin and their effect on plant pathogenic fungi. Sci Rep 8:1–10
Lehn JM (2013) Perspectives in chemistry - steps towards complex matter. Angew Chemie - Int Ed 52:2836–2850
Jin X, Zhu L, Xue B et al (2019) Supramolecular nanoscale drug-delivery system with ordered structure. Natl Sci Rev 6:1128–1137
Bhalla V (2018) Supramolecular chemistry. Resonance 23:277–290
Torresi S, Famulari A, Martí-Rujas J (2020) Kinetically controlled fast crystallization of M12L 8 poly-[ n ]-catenanes using the 2,4,6-Tris(4-pyridyl)benzene ligand and ZnCl2 in an aromatic environment. J Am Chem Soc 142:9537–9543
Marti-Rujas J, Desmedt A, Harris KDM, Guillaume F (2004) Direct time-resolved and spatially resolved monitoring of molecular transport in a crystalline nanochannel system. J Am Chem Soc 126:11124–11125
Martí-Rujas J, Desmedt A, Harris KDM, Guillaume F (2009) Bidirectional transport of guest molecules through the nanoporous tunnel structure of a solid inclusion compound. J Phys Chem C 113:736–743
Couzi M, Guillaume F, Harris KDM (2018) A phenomenological model for structural phase transitions in incommensurate alkane/urea inclusion compounds. R Soc Open Sci 5:180058
Egleston BD, Luzyanin KV, Brand MC et al (2020) Controlling gas selectivity in molecular porous liquids by tuning the cage window size. Angew Chemie Int Ed 59:7362–7366
Abet V, Szczypiński FT, Little MA et al (2020) Inducing social self-sorting in organic cages to tune the shape of the internal cavity. Angew Chemie Int Ed 59:16755–16763
Vepuri SB, Anbazhagan S, Divya D, Padmini D (2013) Review article a review on supramolecular chemistry in drug. Indones JPharm 24:131–150
Geng W-C, Sessler JL, Guo D-S (2020) Supramolecular prodrugs based on host–guest interactions. Chem Soc Rev 49:2303–2315
Assaf KI, Nau WM (2015) Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem Soc Rev 44:394–418
Lee R, Mason SA, Mossou E et al (2016) Neutron diffraction studies on guest-induced distortions in urea inclusion compounds. Cryst Growth Des 16:7175–7185
Zheng B, Wang F, Dong S, Huang F (2012) Supramolecular polymers constructed by crown ether-based molecular recognition. Chem Soc Rev 41:1621–1636
Harada A, Takashima Y, Yamaguchi H (2009) Cyclodextrin-based supramolecular polymers. Chem Soc Rev 38:875
Zhao H-X, Guo D-S, Wang L-H et al (2012) A novel supramolecular ternary polymer with two orthogonal host–guest interactions. Chem Commun 48:11319
Koner AL, Ghosh I, Saleh N, Nau WM (2011) Supramolecular encapsulation of benzimidazole-derived drugs by cucurbit[7]uril. Can J Chem 89:139–147
Kim J, Jung IS, Kim SY et al (2000) New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J Am Chem Soc 122:540–541
Lee JW, Samal S, Selvapalam N et al (2003) Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res 36:621–630
Das D, Assaf KI, Nau WM (2019) Applications of cucurbiturils in medicinal chemistry and chemical biology. Front Chem 7
Jeon WS, Moon K, Park SH et al (2005) Complexation of ferrocene derivatives by the cucurbit[7]uril host: a comparative study of the cucurbituril and cyclodextrin host families. J Am Chem Soc 127:12984–12989
Isaacs L (2009) Cucurbit[n]urils: from mechanism to structure and function. Chem Commun:619–629
Edris AE (2007) Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phyther Res 21:308–323
Sharifi-Rad J, Ozleyen A, Boyunegmez Tumer T et al (2019) Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 9:679
Feyaerts AF, Luyten W, Van Dijck P (2020) Striking essential oil: tapping into a largely unexplored source for drug discovery. Sci Rep 10:2867
Kfoury M, Landy D, Fourmentin S (2018) Characterization of cyclodextrin/volatile inclusion complexes: a review. Molecules 23:1204
Chan W (2014) Cytotoxic effects of dillapiole on embryonic development of mouse blastocysts in vitro and in vivo. Int J Mol Sci 15:10751–10765. https://doi.org/10.3390/ijms150610751
Rojas-Martínez R, Arrieta J, Cruz-Antonio L et al (2013) Dillapiole, isolated from peperomia pellucida, shows gastroprotector activity against ethanol-induced gastric lesions in wistar rats. Molecules 18:11327–11337
Ferreira AK, De-Sá-Júnior PL, Pasqualoto KFM et al (2014) Cytotoxic effects of dillapiole on MDA-MB-231 cells involve the induction of apoptosis through the mitochondrial pathway by inducing an oxidative stress while altering the cytoskeleton network. Biochimie 99:195–207
Parise-Filho R, Pastrello M, Pereira Camerlingo CE et al (2011) The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm Biol 49:1173–1179
Baldi A (2010) Computational approaches for drug design and discovery: an overview. Syst Rev Pharm 1:99
Macalino SJY, Gosu V, Hong S, Choi S (2015) Role of computer-aided drug design in modern drug discovery. Arch Pharm Res 38:1686–1701
de Oliveira VE, Almeida EWC, Castro HV et al (2011) Carotenoids and β-cyclodextrin inclusion complexes: Raman spectroscopy and theoretical investigation. J Phys Chem A 115:8511–8519
Liang Q, Chai K, Lu K et al (2017) Theoretical and experimental studies on the separation of cinnamyl acetate and cinnamaldehyde by adsorption onto a β-cyclodextrin polyurethane polymer. RSC Adv 7:43502–43511
Guendouzi A, Mekelleche SM, Brahim H, Litim K (2017) Quantitative conformational stability host-guest complex of Carvacrol and Thymol with β-cyclodextrin: a theoretical investigation. J Incl Phenom Macrocycl Chem 89:143–155
Lawtrakul L, Inthajak K, Toochinda P (2014) Molecular calculations on β-cyclodextrin inclusion complexes with five essential oil compounds from Ocimum basilicum (sweet basil). ScienceAsia 40:145
Tomar SS, Maheshwari ML, Mukerjee (1979) Syntheses and synergistic activity of some pyrethrum synergists from dillapiole. Agric Biol Chem 43:1479–1483
Parise-Filho R, Pasqualoto KFM, Magri FMM et al (2012) Dillapiole as Antileishmanial agent: discovery, cytotoxic activity and preliminary SAR studies of dillapiole analogues. Arch Pharm (Weinheim) 345:934–944
Ferreira R, Monteiro M, Silva J, Maia J (2016) Antifungal action of the dillapiole-rich oil of Piper aduncum against dermatomycoses caused by filamentous Fungi. Br J Med Med Res 15:1–10
Nascimento LD d, Almeida LQ, Sousa EMP d et al (2020) Microwave-assisted extraction: an alternative to extract Piper aduncum essential oil. Brazilian J Dev 6:40619–40638
Luís D, Cruz V, Sumita TC et al (2020) Effects of the semi-synthetic compound dillapiole n -butyl ether in Balb / C mice. J Toxicol Environ Heal Part A 0:1–12
Salazar LC, Ortiz-Reyes A, Rosero DM, Lobo-Echeverri T (2020) Dillapiole in Piper holtonii as an inhibitor of the symbiotic fungus Leucoagaricus gongylophorus of leaf-cutting ants. J Chem Ecol 46:668–674
Liu SQ, Scott IM, Pelletier Y et al (2014) Dillapiol: a pyrethrum synergist for control of the Colorado potato beetle. J Econ Entomol 107:797–805
Fazolin M, Estrela JLV, Medeiros AFM et al (2016) Synergistic potential of dillapiole-rich essential oil with synthetic pyrethroid insecticides against fall armyworm. Ciência Rural 46:382–388
da Fonseca MS, Domingos PRC, da Silva Pinto AC, Rafael MS (2016) Toxic effect and genotoxicity of the semisynthetic derivatives dillapiole ethyl ether and dillapiole n -butyl ether for control of Aedes albopictus (Diptera: Culicidae). Mutat Res Toxicol Environ Mutagen 807:1–7
Walia S, Saha S, Parmar BS (2004) Liquid chromatographic method for the analysis of two plant based insecticide synergists dillapiole and dihydrodillapiole. J Chromatogr A 1047:229–233
Hayashi Y, Ohara K, Taki R et al (2018) Combined analysis of 1,3-benzodioxoles by crystalline sponge X-ray crystallography and laser desorption ionization mass spectrometry. Analyst 143:1475–1481
Taylor P, Pinalli R, Barboza T et al (2013) Detection of amphetamine precursors with quinoxaline-bridged cavitands. Supramol Chem 25:682–687
Dassault Systèmes BIOVIA (2018) Discovery studio, v19. San Diego, Dassault Systèmes
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision D.01. Gaussian, Inc., Wallingford
Morris GM, Huey R, Lindstrom W et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Morris GM, Goodsell DS, Halliday RS et al (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Rizvi SMD, Shakil S, Haneef M (2013) A simple click by click protocol to perform docking: Autodock 4.2 made easy for non-bioinformaticians. EXCLI J 12:830–857
Ma D, Chan DS-H, Leung C (2011) Molecular docking for virtual screening of natural product databases. Chem Sci 2:1656–1665
Kitchen DB, Decornez H, Furr JR, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 3:935–949
Thangaraj S, Gopalakrishnan VK (2011) Molecular docking and QSAR studies on plant derived bioactive compounds as potent inhibitors of DEK oncoprotein. Asian J Pharm Clin Res 4:67–71
Baker EN, Hubbard RE (1984) Hydrogen bonding in globular proteins. Prog Biophys Mol Biol 44:97–179
Bissantz C, Kuhn B, Stahl M (2010) A medicinal chemist’s guide to molecular interactions. J Med Chem 53:5061–5084
Pierce AC, Sandretto KL, Bemis GW (2002) Kinase inhibitors and the case for CH···O hydrogen bonds in protein-ligand binding. Proteins Struct Funct Genet 49:567–576
Rajendiran N, Sankaranarayanan RK, Saravanan J (2015) Nanochain and vesicles formed by inclusion complexation of 4,4′-diaminobenzanilide with cyclodextrins. J Exp Nanosci 10:880–899
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Steiner T (2002) The hydrogen bond in the solid state. Angew Chemie Int Ed 41:48–76
Masturaini CA (2018) Synthesis of Dillapiole derivatives and their gabaergic, acetylcholinesterase and cytotoxicity activities. Master’s thesis, Universiti Malaysia Terengganu
Funding
We are grateful to the computational chemistry research group at the Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia for the high-performance computer facility provided and Fundamental Research Grant Scheme funding vot. no. 59261.
Author information
Authors and Affiliations
Contributions
Conceptualization: Siti Fatimah Zaharah Mustafa, Siti Rosilah Arsad; Methodology: Siti Fatimah Zaharah Mustafa, Hassan H. Abdallah; Formal analysis and investigation: Siti Fatimah Zaharah Mustafa, Siti Rosilah Arsad, Hassan H. Abdallah; Writing – original draft preparation: Siti Fatimah Zaharah Mustafa, Siti Rosilah Arsad; Writing – review and editing: Habsah Mohamad, Hassan H. Abdallah, Hasmerya Maarof; Funding acquisition: Habsah Mohamad; Resources: Hasmerya Maarof.
Corresponding author
Ethics declarations
Competing for interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Code availability
Discovery Studio, v19, AutoDock 4.2 software and Gaussian 09, Revision D.01.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mustafa, S.F.Z., Arsad, S.R., Mohamad, H. et al. Host-guest molecular encapsulation of cucurbit[7]uril with dillapiole congeners using docking simulation and density functional theory approaches. Struct Chem 32, 1151–1161 (2021). https://doi.org/10.1007/s11224-020-01708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01708-4