Abstract
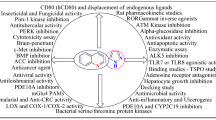
The benzimidazole nucleus is an important pharmacophore in drug discovery, being a good bioisostere of naturally occurring nucleotides. This heterocycle may represent a type of privileged substructure which can interact with proteins and enzymes; it has, hence, been extensively utilized as a drug scaffold in medicinal chemistry. The connection between wide ranging biological activity and compounds containing the benzimidazole nucleus is known, and well documented in the literature. Benzimidazole derivatives have a multitude of interesting pharmacological activity, including antiviral, antitumor, antihypertensive, proton pump inhibitory, anthelmintic, antimicrobial, and anti-inflammatory activity. Accordingly, a brief survey is given below covering the synthesis of 2-phenybenzimidazole derivatives and their biological importance.








Similar content being viewed by others
References
M. Alamgir, D.C. Black, N. Kumar, Synthesis, reactivity and biological activity of benzimidazoles. Top. Heterocycl. Chem. 9, 87–118 (2007)
N. Dege, M. Şekerci, S. Servi, M. Dinçer, Ü. Demirbaş, Structure of 1-(thiophen-2-ylmethyl)-2-(thiophen-2-yl)-1H-benzimidazole. Turk. J. Chem. 30, 103–108 (2006)
G. Ayhan-Kılcıgil, N. Altanlar, Synthesis and antimicrobial activities of some new benzimidazole derivatives. IL Farm. 58, 1345–1350 (2003)
B. Miklaszewski, S. Niememtowski, Vergleichendes studium der drei isomeren (b)-amino-phenylbenzimidazole. Chem. Ber. 34, 2953–2974 (1901)
C. Kus, G. Ayhan-Kılcıgil, M. Tunçbilek, N. Altanlar, T. Çoban, B. Can-Eke, M. Iscan, Synthesis, antimicrobial and antioxidant activities of some benzimidazole derivatives. Lett. Drug Des. Discov. 6, 374–379 (2009)
Y.S. Chhonker, B. Veenu, S.R. Hasim, N. Kaushik, D. Kumar, P. Kumar, Synthesis and pharmacological evaluation of some new 2-phenyl benzimidazoles derivatives and their Schiff’s bases. E. J. Chem. 6, 342–346 (2009)
K. Sreena, R. Ratheesh, M. Rachana, M. Poornima, C. Shyni, Synthesis and anthelmintic activity of benzimidazole derivatives. Hygeia 1, 21–22 (2009)
C. Mei-Sze, S. Dong-Fang, W. Samantha, D.B. Tracey, H. Ian, P.N. Shaw, A.B. David, A.S. Lesley, F.G. Stevens, Antitumor benzothiazoles. 7. Synthesis of 2-(4-acylamino phenyl)benzothiazoles and investigations into the role of acetylation in the antitumor activities of the parent amines. J. Med. Chem. 42, 381–392 (1999)
N.S. Moorthy, R. Dubey, Comparative studies on conventional and microwave assisted synthesis of benzimidazole and their 2-substituted derivative with the effect of salt form of reactant. Chem. Pharm. Bull. 55, 115–117 (2007)
K. Niknam, A. Fatehi-Raviz, Synthesis of 2-substituted benzimidazoles and bis-benzimidazoles by microwave in the presence of alumina-methanesulfonic acid. J. Iran. Chem. Soc. 4, 438–443 (2007)
J.H. Clark, Solid acids for green chemistry. Acc. Chem. Res. 35, 791–797 (2002)
A. Corma, Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 95, 559–614 (1995)
A. Corma, H. Garcia, Lewis acids: from conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem. Rev. 103, 4307–4365 (2003)
T. Okuhara, Water-tolerant solid acid catalysts. Chem. Rev. 102, 3641–3665 (2002)
M.A. Hamer, Q. Sun, Solid acid catalysis using ion-exchange resins. Appl. Catal. A 221, 45–62 (2001)
R.V. Shingalapur, K.M. Hosamani, An efficient and eco-friendly tungstate promoted zirconia (WOx/ZrO2) solid acid catalyst for the synthesis of 2-aryl benzimidazoles. Catal. Lett. 137, 63–68 (2010)
Q. Su, B. Gatto, C. Yu, A. Liu, L.F. Liu, E.J. LaVoie, Synthesis and evaluation of terbenzimidazoles as topoisomerase I inhibitors. J. Med. Chem. 38, 3638–3644 (1995)
Z. Xiao-Kang, G. Jian, T. Yoko, F.B. Kenneth, J.C. Sung, C. Huey-Hwa, C. Yung-Chi, G. Marc, L. Kuo-Hsiung, Antitumor agents. 194. Synthesis and biological evaluations of 4-β-mono-, -di-, and -trisubstituted aniline-4′-o-demethyl-podophyllotoxin and related compounds with improved pharmacological profiles 1. J. Med. Chem. 42, 2441–2446 (1999)
S. Gowda, B.K.K. Gowda, D.C. Gowda, Hydrazinium monoformate: a new hydrogen donor. Selective reduction of nitro compounds catalyzed by commercial zinc dust. Synth. Commun. 33, 281–289 (2003)
M.A. Pagano, M. Andrzejewska, M. Ruzzene, S. Sarno, L. Cesaro, J. Bain, M. Elliott, F. Meggio, Z. Kazimierczuk, L.A. Pinna, Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. J. Med. Chem. 47, 6239–6247 (2004)
M. Tonelli, M. Simone, B. Tasso, F. Novelli, V. Boido, F. Sparatore, G. Paglietti, S. Pricl, G. Giliberti, S. Blois, C. Ibba, G. Sanna, R. Loddo, P. La Colla, Antiviral activity of benzimidazole derivatives. II. Antiviral activity of 2-phenylbenzimidazole derivatives. Bioorg. Med. Chem. 18, 2937–2953 (2010)
S. Alper, Ö.T. Arpaci, E.Ş. Aki, I. Yalcin, Some new bi- and ter-benzimidazole derivatives as topoisomerase I inhibitors. IL Farm. 58, 497–507 (2003)
T. Shunya, K. Hiroyoshi, N. Montowo, Design and synthesis of a heparanase inhibitor with pseudodisaccharide structure. Tetrahedron 57, 6915–6926 (2001)
C.P. Jeffrey, C. Murty, C. Josh, F. Irene, G. Lloyd, G. Daniel, H. Diane, H. Christine, J. James, K. Wenling, S.K. Gregory, T.L. Joseph, M. Charles, M. John, R. John, S. Linda, W. Jay, 2-Phenyl-4-piperazinylbenzimidazoles: orally active inhibitors of the gonadotropin releasing hormone (GnRH) receptor. Bioorg. Med. Chem. 16, 6617–6640 (2008)
E.B. Skibo, W.G. Schulz, Pyrrolo[1,2-a]benzimidazole-based aziridinyl quinones. A new class of DNA cleaving agent exhibiting G and A base specificity. J. Med. Chem. 36, 3050–3055 (1993)
A.R. Katritzky, D.A. Dobchev, D.C. Fara, M. Karelson, QSAR studies on 1-phenyl benzimidazoles as inhibitors of the platelet derived growth factor. Bioorg. Med. Chem. 13, 6598–6608 (2005)
A. Rao, A. Chimirri, E. De Clercq, A.M. Monforte, P. Monforte, C. Pannecouque, M. Zappalà, Synthesis and anti-HIV activity of 1-(2,6-difluorophenyl)-1H,3H-thiazolo[3,4-a]benzimidazole structurally-related 1,2-substituted benzimidazoles. IL Farm. 57, 819–823 (2002)
J.R. Hwu, R. Singha, S.C. Hong, Y.H. Chang, A.R. Das, I. Vliegen, E. De Clerq, J. Neyts, Synthesis of new benzimidazole-coumarin conjugates as anti-hepatitis C virus agents. Antivir. Res. 77, 157–162 (2008)
D.L. Evers, G. Komazin, D. Shin, D.D. Hwang, L.B. Townsend, J.C. Drach, Interactions among antiviral drugs acting late in the replication cycle of human cytomegalovirus. Antivir. Res. 56, 61–72 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khattab, M., Galal, S.A., Ragab, F.A.F. et al. Different synthetic routes to 4-(1H-benzo[d]imidazol-2-yl)aniline. Res Chem Intermed 39, 2917–2923 (2013). https://doi.org/10.1007/s11164-012-0843-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0843-z