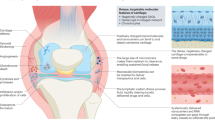
Abstract
Osteoarthritis (OA) is an incapacitating and one of the most common physically degenerative conditions with an assorted etiology and a highly complicated molecular mechanism that to date lacks an efficient treatment. The capacity to design biological networks and accurately modify existing genomic sites holds an apt potential for applications across medical and biotechnological sciences. One of these highly specific genomes editing technologies is the CRISPR/Cas9 mechanism, referred to as the clustered regularly interspaced short palindromic repeats, which is a defense mechanism constituted by CRISPR associated protein 9 (Cas9) directed by small non-coding RNAs (sncRNA) that bind to target DNA through Watson-Crick base pairing rules where subsequent repair of the target DNA is initiated. Up-to-date research has established the effectiveness of the CRISPR/Cas9 mechanism in targeting the genetic and epigenetic alterations in OA by suppressing or deleting gene expressions and eventually distributing distinctive anti-arthritic properties in both in vitro and in vivo osteoarthritic models. This review aims to epitomize the role of this high-throughput and multiplexed gene editing method as an analogous therapeutic strategy that could greatly facilitate the clinical development of OA-related treatments since it’s reportedly an easy, minimally invasive technique, and a comparatively less painful method for osteoarthritic patients.



Similar content being viewed by others
Availability of data and materials
Not applicable.
Change history
22 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11154-023-09867-5
Abbreviations
- CRISPR/Cas9:
-
Clustered Regularly Interspaced Short palindromic repeats and CRISPR-associated protein 9
- OA:
-
Osteoarthritis
- RA:
-
Rheumatoid Arthritis
- MSCs:
-
Mesenchymal Stem Cells
- DNA:
-
Deoxyribonucleic Acid
- gRNA:
-
Guide Ribonucleic Acid
- SBP:
-
Subchondral Bone Plate
- SBT:
-
Subchondral Bone Trabeculae
- SBMLs:
-
Subchondral Bone Marrow Lesions
- SASP:
-
Senescence-Associated Secretory Phenotype
- MMPs:
-
Matrix metalloproteinases
- ECM:
-
Extracellular Matrix
- ACAN:
-
Aggrecan
- TNF:
-
Tumor Necrosis Factor
- TNFα:
-
Tumor Necrosis Factor alpha
- ADAMTS:
-
A Disintegrin and Metalloproteinase with Thrombospondin motifs
- IL:
-
Interleukin
- Th2:
-
T helper cell type 2
- CD4+:
-
Cluster of differentiation 4
- sIL-4R:
-
Soluble interleukin-4 receptor
- sIL-6R:
-
Soluble interleukin-6 receptor
- mIL-6R:
-
Membrane-bound IL-6 receptor
- FLS:
-
Fibroblast-like Synoviocytes
- ADAM17/TACE:
-
A Disintegrin and Metalloprotease 17/Tumor Necrosis Factor-Alpha Converting Enzyme
- CD130/gp130:
-
Glycoprotein 130
- sgp130:
-
Soluble glycoprotein 130
- mgp130:
-
Membrane glycoprotein 130
- SMAD:
-
Small Mothers Against Decapentaplegic
- ERK1/2MAP:
-
Extracellular Signal-regulated Kinase 1/2 Mitogen-activated Protein
- BMP:
-
Bone Morphogenetic Proteins
- TGF-β:
-
Transforming Growth Factor Beta
- NKX3-2:
-
NK3 homeobox 2
- PDGF:
-
Platelet-derived Growth Factor
- VSMCs:
-
Vascular Smooth Muscle Cells
- FGF:
-
Fibroblast Growth Factors
- CDMP-1:
-
Cartilage-Derived Morphogenetic Protein
- ROS:
-
Reactive Oxygen Species
- OH −:
-
Hydroxyl Radical
- H2O2 −:
-
Hydrogen Peroxide
- O2 −:
-
Superoxide Anion
- NO −:
-
Nitric Oxide
- OCl −:
-
Hypochlorite Ion
- NADPH:
-
Nicotinamide Adenine Dinucleotide Phosphate
- LPO:
-
Lipid-based Peroxidation
- LOOH:
-
Lipid Hydroperoxides
- ox-LDL:
-
Oxidized Low-Density Lipoprotein
- NOS:
-
Nitrous Oxide Synthase
- PI3K/Akt:
-
Phosphatidylinositol 3-Kinase / Protein Kinase B
- MEK/ERK:
-
Mitogen-activated Protein Kinase / Extracellular Signal-regulated Kinase
- iNOS:
-
Inducible Nitric Oxide Synthase
- NF-κB:
-
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- PGE2:
-
Prostaglandin E2
- ICE:
-
Interleukin-1beta Converting Enzyme
- NSAIDs:
-
Non-Steroidal Anti-Inflammatory Drugs
- HA:
-
Hyaluronic Acid / Hyaluronate
- PRP:
-
Platelet-Rich Plasma
- BMAC:
-
Bone Marrow Aspirate Concentrate
- AD-MSCs:
-
Adipose-Derived Mesenchymal Stem Cells
- VEGF:
-
Vascular Endothelial Growth Factor
- VAS:
-
Visual Analogue Scale
- WOMAC:
-
The Western Ontario and McMaster Universities Arthritis Index
- SpCas-9:
-
Streptococcus pyogenes Cas9 nuclease
- PAM:
-
Protospacer Adjacent Motif
- RuvC:
-
Recombination UV C resolvase
- HNH:
-
Homing endonuclease
- REC:
-
Recognition lobe
- NUC:
-
Nuclease lobe
- crRNA:
-
Crispr RNA
- sncRNA:
-
Small non-coding RNA
- tracrRNA:
-
Trans-activating Crispr RNA
- DSBs:
-
Double-Stranded Breaks
- HDR:
-
Homology-Directed Repair
- NHEJ:
-
Non-homologous End Joining
- AAV:
-
Adeno-associated virus
- rAAV:
-
Recombinant Adeno-associated virus
- scAAV:
-
Self-complementary recombinant Adeno-associated virus
- cDNA:
-
Complementary DNA
- + ssRNA:
-
Positive-sense single RNA
- TILs:
-
Tumor Infiltrating Lymphocytes
- HSP70:
-
Heat Shock Protein 70
- CHS:
-
Chondroitin Sulfate
- ELMO1:
-
Engulfment and Cell Motility 1
- SOX9:
-
SRY-Box Transcription Factor 9
- USP11:
-
Ubiquitin Specific Peptidase 11
- WNT 16:
-
Int/Wingless Protein
- BGLAP:
-
Bone Gamma-Carboxyglutamate Protein
- miR-140:
-
Micro RNA 140
- NcRNA:
-
Non-coding RNA
- HAS2:
-
Hyaluronan Synthase 2
- STNFR1α:
-
Soluble Tumor Necrosis Factor Receptor 1
- IL-1RA:
-
Interleukin 1 Beta Receptor Antagonist
- PRG4:
-
Proteoglycan 4
- DANCR:
-
Differentiation Antagonizing Non-Protein Coding RNA
- TRPV4:
-
Transient Receptor Potential Cation Channel Subfamily V Member 4
- CX43:
-
Connexin 43
- COL2A1:
-
Collagen, Type II, Alpha 1
- NGF:
-
Nerve Growth Factor
- CBX4:
-
Chromobox 4
- FOXD1:
-
Forkhead Box D1
- YAP:
-
Yes-associated Protein 1
- iPSCs:
-
Induced Pluripotent Stem Cells
- RCS:
-
Rat Chondrosarcoma Cell Line
- ASC:
-
Adipose Stromal Cells
- FDA:
-
The Food and Drug Administration
- CRISPRi:
-
CRISPR interference
- HDAd:
-
Helper-Dependent Adenoviral Vectors
- DGCR8:
-
DiGeorge critical region-8
References
Vina ER, Kwoh CK. Epidemiology of osteoarthritis: Literature update. Curr Opin Rheumatol. 2018;30(2):160–7.
Loeser RF, et al. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707.
Kraus VB, et al. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr Cartil. 2015;23(8):1233–41.
Glyn-Jones S, et al. Osteoarthritis. Lancet. 2015;386(9991):376–87.
Barnett R. Osteoarthritis. Lancet. 2018;391(10134):1985.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–34.
Grassel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9.
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21(9):1145–53.
Bartley EJ, Palit S, Staud R. Predictors of osteoarthritis pain: The importance of resilience. Curr Rheumatol Rep. 2017;19(9):57.
Deveza LA, Nelson AE, Loeser RF. Phenotypes of osteoarthritis: current state and future implications. Clin Exp Rheumatol. 2019;37(Suppl 120):64–72.
Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018;40:59–66.
Diekman BO, Guilak F. Stem cell-based therapies for osteoarthritis: Challenges and opportunities. Curr Opin Rheumatol. 2013;25(1):119–26.
Fitzgerald J. Applications of CRISPR for musculoskeletal research. Bone Joint Res. 2020;9(7):351–9.
Deshpande K, et al. Clustered regularly interspaced short palindromic repeats/Cas9 genetic engineering: Robotic genetic surgery. Am J Robot Surg. 2015;2(1):49–52.
Fan D, et al. Efficient CRISPR/Cas9-mediated targeted mutagenesis in populus in the first generation. Sci Rep. 2015;5:12217.
Kleinstiver BP, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523(7561):481–5.
Tanikella AS, et al. Emerging gene-editing modalities for osteoarthritis. Int J Mol Sci. 2020;21(17).
Zhao L, et al. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis. 2019;78(5):676–82.
Karimian A, et al. CRISPR/Cas9 technology as a potent molecular tool for gene therapy. J Cell Physiol. 2019;234(8):12267–77.
Stewart HL, Kawcak CE. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front Vet Sci. 2018;5:178.
Jang S, Lee K, Ju JH. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int J Mol Sci. 2021;22(5).
Kulkarni P, et al. Pathophysiological landscape of osteoarthritis. Adv Clin Chem. 2021;100:37–90.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59.
Juma SN, et al. Osteoarthritis versus psoriasis arthritis: Physiopathology, cellular signaling, and therapeutic strategies. Genes Dis. 2023.
Walker WT, Kawcak CE, Hill AE. Medial femoral condyle morphometrics and subchondral bone density patterns in Thoroughbred racehorses. Am J Vet Res. 2013;74(5):691–9.
Holmdahl DE, Ingelmark BE. The contact between the articular cartilage and the medullary cavities of the bone. Acta Orthop Scand. 1950;20(2):156–65.
Hu Y, et al. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20.
Zhu X, et al. Subchondral bone remodeling: A therapeutic target for osteoarthritis. Front Cell Dev Biol. 2020;8:607764.
Castañeda S, et al. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–23.
Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–44.
Fan X, et al. Macro, micro, and molecular. Changes of the osteochondral interface in osteoarthritis development. Front Cell Dev Biol. 2021;9:659654.
Pan J, et al. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res. 2009;27(10):1347–52.
Lyons TJ, et al. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet Disord. 2006;7:52.
Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419–33.
Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4(6):221–9.
Perry TA, et al. Association between Bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthr Cartil. 2020;28(3):316–23.
Fusco M, et al. Degenerative joint diseases and neuroinflammation. Pain Pract. 2017;17(4):522–32.
Raynauld JP, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis. 2008;67(5):683–8.
Chen L, et al. Horizontal fissuring at the osteochondral interface: A novel and unique pathological feature in patients with obesity-related osteoarthritis. Ann Rheum Dis. 2020;79(6):811–8.
Coaccioli S et al. Osteoarthritis: New insight on its pathophysiology. J Clin Med. 2022;11(20).
Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: Effect of intraarticular anesthetic. J Rheumatol. 1996;23(6):1031–6.
Crawford RW, et al. Diagnostic value of intra-articular anaesthetic in primary osteoarthritis of the hip. J Bone Joint Surg Br. 1998;80(2):279–81.
Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88(1):69–78.
Buffington AL, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113(1–2):172–84.
Suri S, et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–8.
Ashraf S, et al. Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70(3):523–9.
Mapp PI, et al. Angiogenesis in two animal models of osteoarthritis. Osteoarthr Cartil. 2008;16(1):61–9.
Denk F, Bennett DL, McMahon SB. Nerve growth factor and pain mechanisms. Annu Rev Neurosci. 2017;40:307–25.
Barker PA, et al. Nerve growth factor signaling and its contribution to pain. J Pain Res. 2020;13:1223–41.
Crowley C, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–11.
Drissi I, Woods WA, Woods CG. Understanding the genetic basis of congenital insensitivity to pain. Br Med Bull. 2020;133(1):65–78.
Miller RJ, Malfait AM, Miller RE. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr Cartil. 2020;28(5):562–71.
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3).
Miller RE, et al. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol. 2015;67(11):2933–43.
Raoof R, Willemen H, Eijkelkamp N. Divergent roles of immune cells and their mediators in pain. Rheumatology (Oxford). 2018;57(3):429–40.
Miller RJ, et al. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–49.
Vincent TL. Peripheral pain mechanisms in osteoarthritis. Pain. 2020;161(Suppl 1):S138–46.
Aizah N, Chong PP, Kamarul T. Early alterations of subchondral bone in the rat anterior cruciate ligament transection model of osteoarthritis. Cartilage. 2021;13(2_suppl):1322S-1333S.
Palmer AJ, et al. Non-invasive imaging of cartilage in early osteoarthritis. Bone Joint J. 2013;95-b(6):738–46.
Findlay DM, Atkins GJ. Osteoblast-chondrocyte interactions in osteoarthritis. Curr Osteoporos Rep. 2014;12(1):127–34.
Finnilä MAJ, et al. Mineral crystal thickness in calcified cartilage and subchondral bone in healthy and osteoarthritic human knees. J Bone Miner Res. 2022;37(9):1700–10.
Prasadam I, et al. Mixed cell therapy of bone marrow-derived mesenchymal stem cells and articular cartilage chondrocytes ameliorates osteoarthritis development. Lab Invest. 2018;98(1):106–16.
Salucci S, Falcieri E, Battistelli M. Chondrocyte death involvement in osteoarthritis. Cell Tissue Res. 2022;389(2):159–70.
Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108–13.
van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr Cartil. 2012;20(3):223–32.
Lv Z, et al. The function and behavior of chondrogenic progenitor cells in osteoarthritis. Ann J. 2020;5
Fukui N, et al. Zonal gene expression of chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 2008;58(12):3843–53.
Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3(5):257–64.
Mobasheri A, et al. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80(3):237–44.
Fukui N, et al. Regional differences in chondrocyte metabolism in osteoarthritis: A detailed analysis by laser capture microdissection. Arthritis Rheum. 2008;58(1):154–63.
Kazemi M, Williams JL. Properties of cartilage-subchondral bone junctions: A narrative review with specific focus on the growth plate. Cartilage. 2021;13(2_suppl):16s–33s.
Grässel S, Muschter D. Peripheral nerve fibers and their neurotransmitters in osteoarthritis pathology. Int J Mol Sci. 2017;18(5).
Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390–8.
Hashimoto S, et al. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthr Cartil. 2002;10(3):180–7.
Gelse K, et al. Osteophyte development–molecular characterization of differentiation stages. Osteoarthr Cartil. 2003;11(2):141–8.
Wong SH, Chiu KY, Yan CH. Review article: Osteophytes. J Orthop Surg (Hong Kong). 2016;24(3):403–10.
Barr AJ, et al. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res Ther. 2015;17(1):228.
Olsson SE, Marshall JL, Story E. Osteophytosis of the knee joint in the dog. A sign of instability. Acta Radiol Suppl. 1972;319:165–7.
Zahan OM, et al. The evaluation of oxidative stress in osteoarthritis. Med Pharm Rep. 2020;93(1):12–22.
Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81–90.
Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73.
Lu P, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12).
Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 2014;1(1):8.
Fell HB, et al. The capacity of pig articular cartilage in organ culture to regenerate after breakdown induced by complement-sufficient antiserum to pig erythrocytes. Calcif Tissue Res. 1976;20(1):3–21.
Pratta MA, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278(46):45539–45.
Karsdal MA, et al. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10(3):R63.
Fan M, et al. Impacts of aging on murine cartilage biomechanics and chondrocyte in situ calcium signaling. J Biomech. 2022;144:111336.
Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824(1):133–45.
Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916–26.
Mitchell PG, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–8.
Staebler S, et al. MIA/CD-RAP regulates MMP13 and is a potential new disease-modifying target for osteoarthritis therapy. Cells. 2023;12(2).
Hu Q, Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci. 2021;22(4).
Little CB, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–33.
Hayami T, Kapila YL, Kapila S. MMP-1 (collagenase-1) and MMP-13 (collagenase-3) differentially regulate markers of osteoblastic differentiation in osteogenic cells. Matrix Biol. 2008;27(8):682–92.
Bay-Jensen AC, et al. Biochemical markers of type II collagen breakdown and synthesis are positioned at specific sites in human osteoarthritic knee cartilage. Osteoarthr Cartil. 2008;16(5):615–23.
Rousseau JC, Chapurlat R, Garnero P. Soluble biological markers in osteoarthritis. Ther Adv Musculoskelet Dis. 2021;13:1759720X211040300.
Duclos ME, et al. Significance of the serum CTX-II level in an osteoarthritis animal model: A 5-month longitudinal study. Osteoarthr Cartil. 2010;18(11):1467–76.
De Ceuninck F, Sabatini M, Pastoureau P. Recent progress toward biomarker identification in osteoarthritis. Drug Discov Today. 2011;16(9–10):443–9.
Sharif M, et al. A 5-yr longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis–association with disease progression. Rheumatology (Oxford). 2007;46(6):938–43.
Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330.
Cabral-Pacheco GA, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24).
Wan J, et al. Matrix metalloproteinase 3: A promoting and destabilizing factor in the pathogenesis of disease and cell differentiation. Front Physiol. 2021;12:663978.
Mahmoud RK, et al. Matrix metalloproteinases MMP-3 and MMP-1 levels in sera and synovial fluids in patients with rheumatoid arthritis and osteoarthritis. Ital J Biochem. 2005;54(3–4):248–57.
Sun S, et al. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet Disord. 2014;15:93.
Bau B, et al. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46(10):2648–57.
Kerkelä E, et al. Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. Br J Cancer. 2001;84(5):659–69.
Valdés-Fernández J, et al. Molecular and cellular mechanisms of delayed fracture healing in Mmp10 (Stromelysin 2) knockout mice. J Bone Miner Res. 2021;36(11):2203–13.
Bondeson J, et al. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8(6):R187.
Lee H, et al. Therapeutic single compounds for osteoarthritis treatment. Pharmaceuticals (Basel). 2021;14(2).
Ding L, Guo D, Homandberg GA. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthr Cartil. 2008;16(10):1253–62.
Hwang HS, et al. Fibronectin fragment-induced expression of matrix metalloproteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res Ther. 2015;17:320.
Dunn SL, et al. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthr Cartil. 2016;24(8):1431–40.
Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873.
Perez-Garcia S, et al. Profile of matrix-remodeling proteinases in osteoarthritis: impact of fibronectin. Cells. 2019;9(1).
Ding L, et al. A single blunt impact on cartilage promotes fibronectin fragmentation and upregulates cartilage degrading stromelysin-1/matrix metalloproteinase-3 in a bovine ex vivo model. J Orthop Res. 2014;32(6):811–8.
Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J Cell Biochem. 2011;112(12):3507–14.
Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5(2):94–103.
Fontanil T, et al. Hyalectanase activities by the ADAMTS metalloproteases. Int J Mol Sci. 2021;22(6).
Song RH, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56(2):575–85.
Scavenius C, et al. Matrix-degrading protease ADAMTS-5 cleaves inter-α-inhibitor and releases active heavy chain 2 in synovial fluids from arthritic patients. J Biol Chem. 2019;294(42):15495–504.
Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825–34.
Punzi L, et al. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2(2):e000279.
Bondeson J, et al. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26(1):139–45.
Apte SS. Anti-ADAMTS5 monoclonal antibodies: Implications for aggrecanase inhibition in osteoarthritis. Biochem J. 2016;473(1):e1-4.
Jiang L, et al. ADAMTS5 in osteoarthritis: Biological functions, regulatory network, and potential targeting therapies. Front Mol Biosci. 2021;8:703110.
Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–8.
Santamaria S, et al. Development of a monoclonal anti-ADAMTS-5 antibody that specifically blocks the interaction with LRP1. MAbs. 2017;9(4):595–602.
Dupuis LE, et al. Adamts5(-/-) mice exhibit altered aggrecan proteolytic profiles that correlate with ascending aortic anomalies. Arterioscler Thromb Vasc Biol. 2019;39(10):2067–81.
Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 2015;3(4):177–92.
Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020;2020:8293921.
Hoff P, et al. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37(1):145–51.
Kapoor M, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42.
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459.
Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(11):375.
Dinarello CA. Overview of the interleukin-1 family of ligands and receptors. Semin Immunol. 2013;25(6):389–93.
Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. 2019;53:212–23.
Westacott CI, et al. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthr Cartil. 2000;8(3):213–21.
Ng EK, et al. Human intestinal epithelial and smooth muscle cells are potent producers of IL-6. Mediators Inflamm. 2003;12(1):3–8.
Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990;144(2):499–505.
Estrada K, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501.
Xue J, et al. Tumor necrosis factor-α induces ADAMTS-4 expression in human osteoarthritis chondrocytes. Mol Med Rep. 2013;8(6):1755–60.
López-Armada MJ, et al. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr Cartil. 2006;14(10):1011–22.
Wang ZW, et al. Elevated levels of interleukin-1β, interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor in patients with knee articular cartilage injury. World J Clin Cases. 2019;7(11):1262–9.
Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: The cytokine connection. Cytokine. 2014;70(2):185–93.
Westacott CI, et al. Tumor necrosis factor-alpha receptor expression on chondrocytes isolated from human articular cartilage. J Rheumatol. 1994;21(9):1710–5.
Cope AP, et al. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992;35(10):1160–9.
Chen C, et al. Interleukin-1β and tumor necrosis factor-α increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim Biophys Sin (Shanghai). 2015;47(2):121–9.
Liao CR, et al. Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020;28:101306.
Pfander D, et al. Tenascin and aggrecan expression by articular chondrocytes is influenced by interleukin 1beta: A possible explanation for the changes in matrix synthesis during osteoarthritis. Ann Rheum Dis. 2004;63(3):240–4.
Steenvoorden MM, et al. Fibroblast-like synoviocyte-chondrocyte interaction in cartilage degradation. Clin Exp Rheumatol. 2007;25(2):239–45.
Oo WM, et al. Disease-modifying drugs in osteoarthritis: Current understanding and future therapeutics. Expert Opin Emerg Drugs. 2018;23(4):331–47.
Fortier LA, et al. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–15.
Civinini R, et al. Growth factors in the treatment of early osteoarthritis. Clin Cases Miner Bone Metab. 2013;10(1):26–9.
Zhao Y, et al. PDGF-induced vascular smooth muscle cell proliferation is associated with dysregulation of insulin receptor substrates. Am J Physiol Cell Physiol. 2011;300(6):C1375–85.
Raica M, Cimpean AM. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals (Basel). 2010;3(3):572–99.
Bennett NT, Schultz GS. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am J Surg. 1993;165(6):728–37.
Tan EM, et al. Platelet-derived growth factors-AA and -BB regulate collagen and collagenase gene expression differentially in human fibroblasts. Biochem J. 1995;310(Pt 2):585–8.
Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1–2):1–57.
Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr Cartil. 2006;14(5):403–12.
Kieswetter K, et al. Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation. Endocrine. 1997;6(3):257–64.
Ellman MB, et al. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420(1):82–9.
Ellman MB, et al. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114(4):735–42.
Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16(3):329–43.
Luyten FP. Cartilage-derived morphogenetic protein-1. Int J Biochem Cell Biol. 1997;29(11):1241–4.
Bai X, et al. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325(2):453–60.
Fan J, et al. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010;6(3):1178–85.
Smith P, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: Comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43(5):1156–64.
Diao H, et al. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A. 2009;15(9):2687–98.
Junttila IS. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 2018;9:888.
Fernández-Moreno M, et al. Genetics in osteoarthritis. Curr Genomics. 2008;9(8):542–7.
Stefik D, et al. An insight into osteoarthritis susceptibility: Integration of immunological and genetic background. Bosn J Basic Med Sci. 2021;21(2):155–62.
Molnar V, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021;22(17).
Silvestri T, et al. Elevated serum levels of soluble interleukin-4 receptor in osteoarthritis. Osteoarthr Cartil. 2006;14(7):717–9.
Doi H, et al. Interleukin-4 downregulates the cyclic tensile stress-induced matrix metalloproteinases-13 and cathepsin B expression by rat normal chondrocytes. Acta Med Okayama. 2008;62(2):119–26.
Yeh LA, et al. Interleukin-4, an inhibitor of cartilage breakdown in bovine articular cartilage explants. J Rheumatol. 1995;22(9):1740–6.
Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 2017;37(2–6):181–212.
Guicheux J, et al. Primary human articular chondrocytes, dedifferentiated chondrocytes, and synoviocytes exhibit differential responsiveness to interleukin-4: Correlation with the expression pattern of the common receptor gamma chain. J Cell Physiol. 2002;192(1):93–101.
Pal M, Febbraio MA, Whitham M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol. 2014;92(4):331–9.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295.
Liu S, et al. Cartilage tissue engineering: From proinflammatory and anti‑inflammatory cytokines to osteoarthritis treatments (Review). Mol Med Rep. 2022;25(3).
Rose-John S, et al. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11(5):613–24.
Schumacher N, Rose-John S. ADAM17 activity and IL-6 trans-signaling in inflammation and cancer. Cancers (Basel). 2019;11(11).
Jostock T, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–7.
Narazaki M, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82(4):1120–6.
Akeson G, Malemud CJ. A role for soluble IL-6 receptor in osteoarthritis. J Funct Morphol Kinesiol. 2017;2(3).
Wiegertjes R, van de Loo FAJ, Blaney Davidson EN. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology (Oxford). 2020;59(10):2681–94.
Harmer D, Falank C, Reagan MR. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front Endocrinol (Lausanne). 2018;9:788.
de Hooge AS, et al. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthr Cartil. 2005;13(1):66–73.
van de Loo FA, et al. Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am J Pathol. 1997;151(1):177–91.
Mostafa Mtairag E, et al. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc Res. 2001;49(4):882–90.
Schulze-Tanzil G, et al. Interleukin-10 and articular cartilage: experimental therapeutical approaches in cartilage disorders. Curr Gene Ther. 2009;9(4):306–15.
Wang YS, et al. Osteogenic potential of human calcitonin gene-related peptide alpha gene-modified bone marrow mesenchymal stem cells. Chin Med J (Engl). 2011;124(23):3976–81.
Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94.
Rai MF, et al. A microarray study of articular cartilage in relation to obesity and severity of knee osteoarthritis. Cartilage. 2020;11(4):458–72.
Greenhill CJ, et al. Interleukin-10 regulates the inflammasome-driven augmentation of inflammatory arthritis and joint destruction. Arthritis Res Ther. 2014;16(4):419.
Jansen NW, et al. Interleukin-10 protects against blood-induced joint damage. Br J Haematol. 2008;142(6):953–61.
Wang Y, Lou S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin Med J (Engl). 2001;114(7):723–5.
Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136(22):3715–28.
Zeng L, et al. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16(15):1990–2005.
Akiyama H, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–28.
Alaaeddine N, et al. Inhibition of tumor necrosis factor alpha-induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: Distinct targeting in the signaling pathways. Arthritis Rheum. 1999;42(4):710–8.
Hart PH, et al. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84(4):536–42.
Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27(12):639–45.
Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–44.
Rahmati M, et al. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30.
Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr Cartil. 2003;11(10):747–55.
Altay MA, et al. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int. 2015;35(10):1725–31.
Ertürk C, et al. Paraoxonase-1 activity and oxidative status in patients with knee osteoarthritis and their relationship with radiological and clinical parameters. Scand J Clin Lab Invest. 2012;72(5):433–9.
Stamler JS, Lamas S, Fang FC. Nitrosylation: The prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–83.
Carlo MD Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–30.
Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthr Cartil. 2008;16(4):515–21.
Soran N, et al. Assessment of paraoxonase activities in patients with knee osteoarthritis. Redox Rep. 2008;13(5):194–8.
van Lent PL, et al. NADPH-oxidase-driven oxygen radical production determines chondrocyte death and partly regulates metalloproteinase-mediated cartilage matrix degradation during interferon-gamma-stimulated immune complex arthritis. Arthritis Res Ther. 2005;7(4):R885–95.
Notoya K, et al. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J Immunol. 2000;165(6):3402–10.
Rosa SC, et al. Nitric oxide synthase isoforms and NF-kappaB activity in normal and osteoarthritic human chondrocytes: Regulation by inducible nitric oxide. Nitric Oxide. 2008;19(3):276–83.
Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12(9):440–50.
Frenkel SR, et al. Effects of nitric oxide on chondrocyte migration, adhesion, and cytoskeletal assembly. Arthritis Rheum. 1996;39(11):1905–12.
Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–91.
Drevet S, et al. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp Gerontol. 2018;111:107–17.
Liu L, et al. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front Mol Biosci. 2022;9:1001212.
Ahmad N, Ansari MY, Haqqi TM. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J Cell Physiol. 2020;235(10):6366–76.
Balaganur V, et al. Effect of S-methylisothiourea, an inducible nitric oxide synthase inhibitor, in joint pain and pathology in surgically induced model of osteoarthritis. Connect Tissue Res. 2014;55(5–6):367–77.
Suantawee T, et al. Upregulation of inducible nitric oxide synthase and nitrotyrosine expression in primary knee osteoarthritis. J Med Assoc Thai. 2015;98(Suppl 1):S91–7.
Watt FE, Gulati M. New drug treatments for osteoarthritis: What is on the horizon? Eur Med J Rheumatol. 2017;2(1):50–8.
Chen L, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–18.
Pelletier JP, et al. The increased synthesis of inducible nitric oxide inhibits IL-1ra synthesis by human articular chondrocytes: Possible role in osteoarthritic cartilage degradation. Osteoarthr Cartil. 1996;4(1):77–84.
Boileau C, et al. The in situ up-regulation of chondrocyte interleukin-1-converting enzyme and interleukin-18 levels in experimental osteoarthritis is mediated by nitric oxide. Arthritis Rheum. 2002;46(10):2637–47.
Henrotin YE, et al. Nitric oxide downregulates interleukin 1beta (IL-1beta) stimulated IL-6, IL-8, and prostaglandin E2 production by human chondrocytes. J Rheumatol. 1998;25(8):1595–601.
Ginn-Pease ME, Whisler RL. Redox signals and NF-kappaB activation in T cells. Free Radic Biol Med. 1998;25(3):346–61.
Lewis R, et al. Strategies for optimising musculoskeletal health in the 21(st) century. BMC Musculoskelet Disord. 2019;20(1):164.
Qin J, et al. Lifetime risk of symptomatic hand osteoarthritis: The Johnston County Osteoarthritis Project. Arthritis Rheumatol. 2017;69(6):1204–12.
DeJulius CR, et al. Recent advances in clinical translation of intra-articular osteoarthritis drug delivery systems. Adv Ther (Weinh). 2021;4(1).
Shuborna NS, et al. Generation of novel hyaluronic acid biomaterials for study of pain in third molar intervention: A review. J Dent Anesth Pain Med. 2019;19(1):11–9.
Browne JA, et al. Stem cells and platelet-rich plasma injections for advanced hip and knee arthritis: Enthusiasm outpaces science. J Arthroplasty. 2019;34(6):1049–50.
Cole BJ, Redondo ML, Cotter EJ. Articular cartilage injuries of the knee: patient health literacy, expectations for management, and clinical outcomes. Cartilage. 2021;12(2):139–45.
Delanois RE, et al. Biologic therapies for the treatment of knee osteoarthritis. J Arthroplasty. 2019;34(4):801–13.
Southworth TM, et al. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32(1):37–45.
Sherman BJ, et al. The role of orthobiologics in the management of osteoarthritis and focal cartilage defects. Orthopedics. 2019;42(2):66–73.
van Buul GM, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362–70.
Dai WL, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: A meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659-670.e1.
Glynn LG, et al. Platelet-rich plasma (PRP) therapy for knee arthritis: A feasibility study in primary care. Pilot Feasibility Stud. 2018;4:93.
Johal H, et al. Impact of platelet-rich plasma use on pain in orthopaedic surgery: A systematic review and meta-analysis. Sports Health. 2019;11(4):355–66.
Cotter EJ, et al. Bone marrow aspirate concentrate for cartilage defects of the knee: From bench to bedside evidence. Cartilage. 2018;9(2):161–70.
Saltzman BM, et al. Stem cells in orthopedics: A comprehensive guide for the general orthopedist. Am J Orthop (Belle Mead NJ). 2016;45(5):280–326.
Huh SW, et al. Autologous bone-marrow mesenchymal cell induced chondrogenesis (MCIC). J Clin Orthop Trauma. 2016;7(3):153–6.
Chahla J, et al. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: A systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
Holton J, et al. The basic science of bone marrow aspirate concentrate in chondral injuries. Orthop Rev (Pavia). 2016;8(3):6659.
Southworth TM, et al. Orthobiologics for focal articular cartilage defects. Clin Sports Med. 2019;38(1):109–22.
Kim JD, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505–11.
Panchal J, Malanga G, Sheinkop M. Safety and efficacy of percutaneous injection of lipogems micro-fractured adipose tissue for osteoarthritic knees. Am J Orthop (Belle Mead NJ). 2018;47(11).
Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: State of the art and lipogems® technology development. Curr Stem Cell Rep. 2016;2(3):304–12.
Jo CH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66.
Schiavone Panni A, et al. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: Identification of a subpopulation with greater response. Int Orthop. 2019;43(1):7–13.
Krawczenko A, Klimczak A. Adipose tissue-derived mesenchymal stem/stromal cells and their contribution to angiogenic processes in tissue regeneration. Int J Mol Sci. 2022;23(5).
Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
Hille F, Charpentier E. CRISPR-Cas: Biology, mechanisms and relevance. Philos Trans R Soc Lond B Biol Sci. 2016;371(1707).
Ishino Y, Krupovic M, Forterre P. History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J Bacteriol. 2018;200(7).
Rath D, et al. The CRISPR-Cas immune system: Biology, mechanisms and applications. Biochimie. 2015;117:119–28.
Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Makarova KS, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015:722–36.
Mohanraju P, et al. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 2016;353(6299):aad5147.
Shmakov S, et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15(3):169–82.
Charpentier E. CRISPR-Cas9: how research on a bacterial RNA-guided mechanism opened new perspectives in biotechnology and biomedicine. EMBO Mol Med. 2015;7(4):363–5.
Asmamaw M, Zawdie B. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biologics. 2021;15:353–61.
Liu Z, et al. Application of different types of CRISPR/Cas-based systems in bacteria. Microb Cell Fact. 2020;19(1):172.
Mei Y, et al. Recent progress in CRISPR/Cas9 technology. J Genet Genomics. 2016;43(2):63–75.
Nishimasu H, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156(5):935–49.
Gasiunas G, et al. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109(39):E2579–86.
Faure G, et al. Comparative genomics and evolution of trans-activating RNAs in Class 2 CRISPR-Cas systems. RNA Biol. 2019;16(4):435–48.
Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23.
Wu X, Kriz AJ, Sharp PA. Target specificity of the CRISPR-Cas9 system. Quant Biol. 2014;2(2):59–70.
Ceasar SA, et al. Insert, remove or replace: A highly advanced genome editing system using CRISPR/Cas9. Biochim Biophys Acta. 2016;1863(9):2333–44.
Westra ER, et al. Type I-E CRISPR-cas systems discriminate target from non-target DNA through base pairing-independent PAM recognition. PLoS Genet. 2013;9(9):e1003742.
Shah SA, et al. Protospacer recognition motifs: Mixed identities and functional diversity. RNA Biol. 2013;10(5):891–9.
Jiang F, Doudna JA. CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29.
Liu M, et al. Methodologies for improving HDR efficiency. Front Genet. 2018;9:691.
Stinson BM, Loparo JJ. Repair of DNA double-strand breaks by the nonhomologous end joining pathway. Annu Rev Biochem. 2021;90:137–64.
Yang H, et al. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. Int J Mol Sci. 2020;21(18).
Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44:113–39.
Wilbie D, Walther J, Mastrobattista E. Delivery aspects of CRISPR/Cas for in vivo genome editing. Acc Chem Res. 2019;52(6):1555–64.
Hui SW. Overview of drug delivery and alternative methods to electroporation. Methods Mol Biol. 2008;423:91–107.
Zavvar M, et al. Gene therapy in rheumatoid arthritis: Strategies to select therapeutic genes. J Cell Physiol. 2019;234(10):16913–24.
Naso MF, et al. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31(4):317–34.
Mingozzi F, High KA. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood. 2013;122(1):23–36.
Gruntman AM, Flotte TR. The rapidly evolving state of gene therapy. Faseb J. 2018;32(4):1733–40.
Nixon AJ, et al. Gene therapy in musculoskeletal repair. Ann N Y Acad Sci. 2007;1117:310–27.
Chen X, Gonçalves MA. Engineered viruses as genome editing devices. Mol Ther. 2016;24(3):447–57.
Yoon DS, et al. Cellular and tissue selectivity of AAV serotypes for gene delivery to chondrocytes and cartilage. Int J Med Sci. 2021;18(15):3353–60.
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358–78.
Watson-Levings RS, et al. Gene therapy in orthopaedics: progress and challenges in pre-clinical development and translation. Front Bioeng Biotechnol. 2022;10:901317.
Kay JD, et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med. 2009;11(7):605–14.
Rey-Rico A, et al. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol Pharm. 2018;15(7):2816–26.
Weimer A, et al. Benefits of recombinant adeno-associated virus (rAAV)-mediated insulinlike growth factor I (IGF-I) overexpression for the long-term reconstruction of human osteoarthritic cartilage by modulation of the IGF-I axis. Mol Med. 2012;18(1):346–58.
Ueblacker P, et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials. 2007;28(30):4480–7.
Morgan MA, et al. Retroviral gene therapy in Germany with a view on previous experience and future perspectives. Gene Ther. 2021;28(9):494–512.
Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–80.
Rosenberg SA, et al. Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323(9):570–8.
Meyerrose TE, et al. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26(7):1713–22.
Gouze E, et al. In vivo gene delivery to synovium by lentiviral vectors. Mol Ther. 2002;5(4):397–404.
Cesana D, et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol Ther. 2014;22(4):774–85.
Patil S, et al. The development of functional non-viral vectors for gene delivery. Int J Mol Sci. 2019;20(21).
Grossin L, et al. Direct gene transfer into rat articular cartilage by in vivo electroporation. Faseb J. 2003;17(8):829–35.
Ohashi S, et al. Successful genetic transduction in vivo into synovium by means of electroporation. Biochem Biophys Res Commun. 2002;293(5):1530–5.
Grossin L, et al. Gene transfer with HSP 70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. Faseb J. 2006;20(1):65–75.
Caplen NJ, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1(1):39–46.
Stern M, et al. The effects of jet nebulisation on cationic liposome-mediated gene transfer in vitro. Gene Ther. 1998;5(5):583–93.
Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7(4):286–91.
Cai Y, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-β1. Bioact Mater. 2023;19:444–57.
Lu H, et al. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE. 2014;9(1):e84703.
Chen Z, et al. Novel nano-microspheres containing chitosan, hyaluronic acid, and chondroitin sulfate deliver growth and differentiation factor-5 plasmid for osteoarthritis gene therapy. J Zhejiang Univ Sci B. 2018;19(12):910–23.
Wu X, et al. Extracellular vesicles: Potential role in osteoarthritis regenerative medicine. J Orthop Translat. 2020;21:73–80.
Gallego-Perez D, et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat Nanotechnol. 2017;12(10):974–9.
Zhou Q, et al. Exosomes in osteoarthritis and cartilage injury: Advanced development and potential therapeutic strategies. Int J Biol Sci. 2020;16(11):1811–20.
Liu Y, et al. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am J Sports Med. 2022;50(4):1088–105.
Liang Y, et al. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12(11):4866–78.
Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther. 2018;29(1):2–14.
Goodrich LR, et al. scAAVIL-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther. 2015;22(7):536–45.
Izal I, et al. IGF-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg. 2008;128(2):239–47.
Wu Y, et al. Circular RNA circPDE4D protects against osteoarthritis by binding to miR-103a-3p and regulating FGF18. Mol Ther. 2021;29(1):308–23.
Cherian JJ, et al. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr Cartil. 2015;23(12):2109–18.
Ha CW, et al. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14(2):247–56.
Geng Y, et al. Intra-articular injection of hUC-MSCs expressing miR-140-5p induces cartilage self-repairing in the rat osteoarthritis. J Bone Miner Metab. 2020;38(3):277–88.
Lee SY, et al. The therapeutic effect of STAT3 signaling-suppressed MSC on pain and articular cartilage damage in a rat model of monosodium iodoacetate-induced osteoarthritis. Front Immunol. 2018;9:2881.
Nejadnik H, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–6.
Li KC, Hu YC. Cartilage tissue engineering: Recent advances and perspectives from gene regulation/therapy. Adv Healthc Mater. 2015;4(7):948–68.
Lee H, et al. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology. 2020;28(5):1237–52.
Lee B, et al. Results of a phase II study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1. J Knee Surg. 2020;33(2):167–72.
Liu C, et al. Delivering metal ions by nanomaterials: Turning metal ions into drug-like cancer theranostic agents. Coord Chem Rev. 2023;494:215332.
Lee MC, et al. A placebo-controlled randomised trial to assess the effect of TGF-ß1-expressing chondrocytes in patients with arthritis of the knee. Bone Joint J. 2015;97-b(7):924–32.
Ha CW, et al. A multicenter, single-blind, phase iia clinical trial to evaluate the efficacy and safety of a cell-mediated gene therapy in degenerative knee arthritis patients. Hum Gene Ther Clin Dev. 2015;26(2):125–30.
Adkar SS, et al. Step-wise chondrogenesis of human induced pluripotent stem cells and purification via a reporter allele generated by CRISPR-Cas9 genome editing. Stem Cells. 2019;37(1):65–76.
Attia N, et al. Mesenchymal stem cells as a gene delivery tool: Promise, problems, and prospects. Pharmaceutics. 2021;13(6).
Farhang N, et al. (*) CRISPR-based epigenome editing of cytokine receptors for the promotion of cell survival and tissue deposition in inflammatory environments. Tissue Eng Part A. 2017;23(15–16):738–49.
Karlsen TA, et al. Generation of IL1β-resistant chondrocytes using CRISPR-CAS genome editing. Osteoarthr Cartil. 2016;24:S325.
Dooley C, Murphy M. Using CRISPR/Cas9 gene editting systems to understand the function of interleukin 16 in progression of osteoarthritis. Osteoarthr Cartil. 2021;29:S2–3.
Dooley C. Investigation of interleukin 16 in osteoarthritis progression through CRISPR/CAS9. Osteoarthr Cartil. 2020;28:S522–3.
Fan Y, et al. CRISPR-Cas9-mediated loss of function of β-catenin attenuates intervertebral disc degeneration. Mol Ther Nucleic Acids. 2022;28:387–96.
Freitas GP, et al. Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Ther. 2021;28(12):748–59.
Arandjelovic S, et al. ELMO1 signaling is a promoter of osteoclast function and bone loss. Nat Commun. 2021;12(1):4974.
Truong VA, et al. CRISPRai for simultaneous gene activation and inhibition to promote stem cell chondrogenesis and calvarial bone regeneration. Nucleic Acids Res. 2019;47(13): e74.
Kaushal K, et al. Genome-wide CRISPR/Cas9-based screening for deubiquitinase subfamily identifies ubiquitin-specific protease 11 as a novel regulator of osteogenic differentiation. Int J Mol Sci. 2022;23(2).
McGowan LM, et al. Wnt16 elicits a protective effect against fractures and supports bone repair in zebrafish. JBMR Plus. 2021;5(3):e10461.
Lambert LJ, et al. Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology. Dis Model Mech. 2016;9(10):1169–79.
Chaudhry N, et al. Highly efficient CRISPR-Cas9-mediated editing identifies novel mechanosensitive microRNA-140 targets in primary human articular chondrocytes. Osteoarthr Cartil. 2022;30(4):596–604.
Huang Y, et al. CRISPR/Cas9 knockout of HAS2 in rat chondrosarcoma chondrocytes demonstrates the requirement of hyaluronan for aggrecan retention. Matrix Biol. 2016;56:74–94.
Brunger JM, et al. Genome engineering of stem cells for autonomously regulated, closed-loop delivery of biologic drugs. Stem Cell Rep. 2017;8(5):1202–13.
Bloquel C, et al. Gene therapy of collagen-induced arthritis by electrotransfer of human tumor necrosis factor-alpha soluble receptor I variants. Hum Gene Ther. 2004;15(2):189–201.
Khakshooy A, Balenton N, Chiappelli F. Lubricin: A principal modulator of the psychoneuroendocrine - osteoimmune interactome - implications for novel treatments of osteoarthritic pathologies. Bioinformation. 2017;13(10):343–6.
Szychlinska MA, et al. Altered joint tribology in osteoarthritis: Reduced lubricin synthesis due to the inflammatory process. New horizons for therapeutic approaches. Ann Phys Rehabil Med. 2016;59(3):149–56.
Waller KA, et al. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013;110(15):5852–7.
Rice SJ, et al. Identification of a novel, methylation-dependent, RUNX2 regulatory region associated with osteoarthritis risk. Hum Mol Genet. 2018;27(19):3464–74.
Liao L, et al. Deletion of Runx2 in articular chondrocytes decelerates the progression of DMM-induced osteoarthritis in adult mice. Sci Rep. 2017;7(1):2371.
Ye Y, Baek SH, Zhang T. Proteomic characterization of endogenous substrates of mammalian ubiquitin ligase Hrd1. Cell Biosci. 2018;8:46.
Nguyen NTK, et al. CRISPR activation of long non-coding RNA DANCR promotes bone regeneration. Biomaterials. 2021;275:120965.
Seidl CI, Fulga TA, Murphy CL. CRISPR-Cas9 targeting of MMP13 in human chondrocytes leads to significantly reduced levels of the metalloproteinase and enhanced type II collagen accumulation. Osteoarthr Cartil. 2019;27(1):140–7.
Nonaka K, et al. Novel gain-of-function mutation of TRPV4 associated with accelerated chondrogenic differentiation of dental pulp stem cells derived from a patient with metatropic dysplasia. Biochem Biophys Rep. 2019;19:100648.
Varela-Eirin M, et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 2018;9(12):1166.
Dicks A, et al. Prospective isolation of chondroprogenitors from human iPSCs based on cell surface markers identified using a CRISPR-Cas9-generated reporter. Stem Cell Res Ther. 2020;11(1):66.
Ren X, et al. Maintenance of nucleolar homeostasis by CBX4 alleviates senescence and osteoarthritis. Cell Rep. 2019;26(13):3643-3656.e7.
Fu L, et al. Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biol. 2019;17(4):e3000201.
Yang M, et al. CRISPR/Cas9 mediated generation of stable chondrocyte cell lines with targeted gene knockouts; analysis of an aggrecan knockout cell line. Bone. 2014;69:118–25.
Klein JC, et al. Functional testing of thousands of osteoarthritis-associated variants for regulatory activity. Nat Commun. 2019;10(1):2434.
Wang W, et al. Positive feedback regulation between USP15 and ERK2 inhibits osteoarthritis progression through TGF-β/SMAD2 signaling. Arthritis Res Ther. 2021;23(1):84.
Chapman K, et al. A meta-analysis of European and Asian cohorts reveals a global role of a functional SNP in the 5’ UTR of GDF5 with osteoarthritis susceptibility. Hum Mol Genet. 2008;17(10):1497–504.
Egli RJ, et al. Functional analysis of the osteoarthritis susceptibility-associated GDF5 regulatory polymorphism. Arthritis Rheum. 2009;60(7):2055–64.
Ala-Kokko L, et al. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990;87(17):6565–8.
Yang Y, et al. A connexin43/YAP axis regulates astroglial-mesenchymal transition in hemoglobin induced astrocyte activation. Cell Death Differ. 2018;25(10):1870–84.
Huynh NP, et al. Long non-coding RNA GRASLND enhances chondrogenesis via suppression of the interferon type II signaling pathway. Elife. 2020;9.
Bonato A, et al. Engineering inflammation-resistant cartilage: bridging gene therapy and tissue engineering. Adv Healthc Mater. 2023;12(17):e2202271.
Orth P, et al. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2119–30.
Deng L, et al. Stabilizing heterochromatin by DGCR8 alleviates senescence and osteoarthritis. Nat Commun. 2019;10(1):3329.
Lu CH, et al. Regenerating cartilages by engineered ASCs: Prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther. 2014;22(1):186–95.
Ko JY, et al. SOX-6, 9-transfected adipose stem cells to treat surgically-induced osteoarthritis in goats. Tissue Eng Part A. 2019;25(13–14):990–1000.
Li X, et al. Species-specific biological effects of FGF-2 in articular cartilage: Implication for distinct roles within the FGF receptor family. J Cell Biochem. 2012;113(7):2532–42.
Li Y, et al. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23(2):266–74.
Gigout A, et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr Cartil. 2017;25(11):1858–67.
Zhang X, Mao Z, Yu C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004;22(4):742–50.
Cucchiarini M, et al. Effects of TGF-β overexpression via rAAV gene transfer on the early repair processes in an osteochondral defect model in minipigs. Am J Sports Med. 2018;46(8):1987–96.
Martinez-Redondo P, et al. αKLOTHO and sTGFβR2 treatment counteract the osteoarthritic phenotype developed in a rat model. Protein Cell. 2020:219–26.
Xu Z, et al. Danshensu inhibits the IL-1β-induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway. Mol Med. 2021;27(1):80.
Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D662–70.
Lee AS, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–7.
Tardif G, et al. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999;42(6):1147–58.
Johnson K, Terkeltaub R. Upregulated ank expression in osteoarthritis can promote both chondrocyte MMP-13 expression and calcification via chondrocyte extracellular PPi excess. Osteoarthr Cartil. 2004;12(4):321–35.
Shimo T, et al. Expression and role of IL-1β signaling in chondrocytes associated with retinoid signaling during fracture healing. Int J Mol Sci. 2020;21(7).
O’Geen H, et al. Ezh2-dCas9 and KRAB-dCas9 enable engineering of epigenetic memory in a context-dependent manner. Epigenetics Chromatin. 2019;12(1):26.
Alves E, et al. Reprogramming the anti-tumor immune response via CRISPR genetic and epigenetic editing. Mol Ther Methods Clin Dev. 2021;21:592–606.
Nishimasu H, et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361(6408):1259–62.
Li T, et al. CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduct Target Ther. 2023;8(1):36.
Gao L, et al. Engineered Cpf1 variants with altered PAM specificities. Nat Biotechnol. 2017;35(8):789–92.
Miller SM, et al. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat Biotechnol. 2020;38(4):471–81.
Walton RT, et al. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368(6488):290–6.
Kim HK, et al. Deep learning improves prediction of CRISPR-Cpf1 guide RNA activity. Nat Biotechnol. 2018;36(3):239–41.
Cullot G, et al. CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat Commun. 2019;10(1):1136.
Yang Y, et al. CRISPR/Cas: advances, limitations, and applications for precision cancer research. Front Med (Lausanne). 2021;8:649896.
Strecker J, et al. Engineering of CRISPR-Cas12b for human genome editing. Nat Commun. 2019;10(1):212.
Moon SB, et al. Improving CRISPR genome editing by engineering guide RNAs. Trends Biotechnol. 2019;37(8):870–81.
Gleditzsch D, et al. PAM identification by CRISPR-Cas effector complexes: Diversified mechanisms and structures. RNA Biol. 2019;16(4):504–17.
Zhang X-H, et al. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucleic Acids. 2015;4:e264.
Gaudelli NM, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017;551(7681):464–71.
Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
Charlesworth CT, et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25(2):249–54.
Kim S, et al. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 2018;28(3):367–73.
Gyngell C, Douglas T, Savulescu J. The ethics of germline gene editing. J Appl Philos. 2017;34(4):498–513.
O’Brien J, et al. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402.
Ramos YF, Meulenbelt I. The role of epigenetics in osteoarthritis: current perspective. Curr Opin Rheumatol. 2017;29(1):119–29.
Lee WY, Wang B. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J Orthop Translat. 2017;9:76–88.
Li S, Huang L. Nonviral gene therapy: Promises and challenges. Gene Ther. 2000;7(1):31–4.
Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9(1):Ge01-6.
Mobasheri A, et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr Opin Rheumatol. 2019;31(1):80–9.
Lim CL, et al. Immunogenicity and immunomodulatory effects of the human chondrocytes, hChonJ. BMC Musculoskelet Disord. 2017;18(1):199.
Greco KV, et al. High density micromass cultures of a human chondrocyte cell line: A reliable assay system to reveal the modulatory functions of pharmacological agents. Biochem Pharmacol. 2011;82(12):1919–29.
Uzieliene I, et al. Non-viral gene therapy for osteoarthritis. Front Bioeng Biotechnol. 2020;8:618399.
Mashel TV, et al. Overcoming the delivery problem for therapeutic genome editing: Current status and perspective of non-viral methods. Biomaterials. 2020;258:120282.
Li X, et al. Nanoparticle-cartilage interaction: Pathology-based intra-articular drug delivery for osteoarthritis therapy. Nanomicro Lett. 2021;13(1):149.
Funding
This work was supported by grants by Zhejiang Provincial Natural Science Foundation of China (LZ23H140001), Zhejiang Qianjiang Talent Program (21040040-E), a startup grant from Zhejiang Sci-Tech University (18042290-Y), the Fundamental Research Funds of Zhejiang Sci-Tech University (2021Q031), funds from National Natural Science Foundation of China (81900806, 81400489), Jiaxing Science Technology Foundation (2023AY11045, 2020AY10001), Zhejiang Health Foundation (2022507032), Social Development Public Welfare Foundation of Ningbo (No.202002N3150, 2022S035), Innovation Project of Distinguished Medical Team in Ningbo (No.2022020405). We apologize to the many researchers whose work could not be cited due to space limitations.
Author information
Authors and Affiliations
Contributions
RV: data analysis, literature formation. XZ, HL: literature formation, supervision, GC: literature formation, supervision, and grant holder.
Corresponding authors
Ethics declarations
Ethics approval
Ethics approval and consent to participate: not applicable.
Consent for publication
All the authors agree current state of the manuscript to be submitted to the journal.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vlashi, R., Zhang, X., Li, H. et al. Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing. Rev Endocr Metab Disord 25, 339–367 (2024). https://doi.org/10.1007/s11154-023-09860-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09860-y