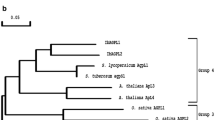
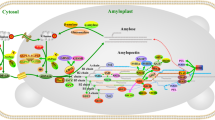
Abstract
Key message
This review outlines research performed in the last two decades on the structural, kinetic, regulatory and evolutionary aspects of ADP-glucose pyrophosphorylase, the regulatory enzyme for starch biosynthesis.
Abstract
ADP-glucose pyrophosphorylase (ADP-Glc PPase) catalyzes the first committed step in the pathway of glycogen and starch synthesis in bacteria and plants, respectively. Plant ADP-Glc PPase is a heterotetramer allosterically regulated by metabolites and post-translational modifications. In this review, we focus on the three-dimensional structure of the plant enzyme, the amino acids that bind the regulatory molecules, and the regions involved in transmitting the allosteric signal to the catalytic site. We provide a model for the evolution of the small and large subunits, which produce heterotetramers with distinct catalytic and regulatory properties. Additionally, we review the various post-translational modifications observed in ADP-Glc PPases from different species and tissues. Finally, we discuss the subcellular localization of the enzyme found in grain endosperm from grasses, such as maize and rice. Overall, this work brings together research performed in the last two decades to better understand the multiple mechanisms involved in the regulation of ADP-Glc PPase. The rational modification of this enzyme could improve the yield and resilience of economically important crops, which is particularly important in the current scenario of climate change and food shortage.

Adapted from Figueroa et al. (2016)

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Asencion Diez MD, Demonte AMM, Guerrero SAA, Ballicora MAA, Iglesias AAA, Asención Diez MD, Demonte AMM, Guerrero SAA, Ballicora MAA, Iglesias AAA (2013) The ADP-glucose pyrophosphorylase from Streptococcus mutans provides evidence for the regulation of polysaccharide biosynthesis in Firmicutes. Mol Microbiol 90:1011–1027. https://doi.org/10.1111/mmi.12413
Asencion Diez MD, Figueroa CM, Esper MC, Mascarenhas R, Aleanzi MC, Liu D, Ballicora MA, Iglesias AA (2020) On the simultaneous activation of Agrobacterium tumefaciens ADP-glucose pyrophosphorylase by pyruvate and fructose 6-phosphate. Biochimie 171–172:23–30. https://doi.org/10.1016/j.biochi.2020.01.012
Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54:207–233. https://doi.org/10.1146/annurev.arplant.54.031902.134927
Ballicora MA, Laughlin MJ, Fu Y, Okita TW, Barry GF, Preiss J (1995) Adenosine 5′-diphosphate-glucose pyrophosphorylase from potato tuber. Significance of the N terminus of the small subunit for catalytic properties and heat stability. Plant Physiol 109:245–251. https://doi.org/10.1104/pp.109.1.245
Ballicora MA, Fu Y, Nesbitt NM, Preiss J (1998) ADP-glucose pyrophosphorylase from potato tubers. Site-directed mutagenesis studies of the regulatory sites. Plant Physiol 118:265–274. https://doi.org/10.1104/PP.118.1.265
Ballicora MA, Frueauf JB, Fu Y, Schürmann P, Preiss J (2000) Activation of the potato tuber ADP-glucose pyrophosphorylase by thioredoxin. J Biol Chem 275:1315–1320. https://doi.org/10.1074/jbc.275.2.1315
Ballicora MA, Iglesias AA, Preiss J (2003) ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol Mol Biol Rev. https://doi.org/10.1128/MMBR.67.2.213-225.2003
Ballicora MA, Iglesias AA, Preiss J (2004) ADP-glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynth Res 79:1–24. https://doi.org/10.1023/B:PRES.0000011916.67519.58
Ballicora MA, Dubay JR, Devillers CH, Preiss J (2005) Resurrecting the ancestral enzymatic role of a modulatory subunit. J Biol Chem 280:10189–10195. https://doi.org/10.1074/jbc.M413540200
Barchiesi J, Hedin N, Iglesias AA, Gomez-Casati DF, Ballicora MA, Busi MV (2017) Identification of a novel starch synthase III from the picoalgae Ostreococcus tauri. Biochimie. https://doi.org/10.1016/j.biochi.2016.12.003
Baris I, Tuncel A, Ozber N, Keskin O, Kavakli IH (2009) Investigation of the interaction between the large and small subunits of potato ADP-glucose pyrophosphorylase. PLoS Comput Biol 5:e1000546. https://doi.org/10.1371/journal.pcbi.1000546
Beaman T, Binder D, Blanchard J, Roderick S (1997) Three-dimensional structure of tetrahydrodipicolinate N-succinyltransferase. Biochemistry 36:489–494. https://doi.org/10.1021/BI962522Q
Beckles DM, Smith AM, ap Rees T (2001) A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs. Plant Physiol 125:818–827. https://doi.org/10.1104/pp.125.2.818
Bhayani JA, Hill BL, Sharma A, Iglesias AA, Olsen KW, Ballicora MA (2019) Mapping of a regulatory site of the Escherichia coli ADP-glucose pyrophosphorylase. Front Mol Biosci. https://doi.org/10.3389/FMOLB.2019.00089
Blankenfeldt W, Asuncion M, Lam JS, Naismith JH (2000) The structural basis of the catalytic mechanism and regulation of glucose-1-phosphate thymidylyltransferase (RmlA). EMBO J 19:6652. https://doi.org/10.1093/EMBOJ/19.24.6652
Boehlein SK, Shaw JR, Stewart JD, Hannah LC (2008) Heat stability and allosteric properties of the maize endosperm ADP-glucose pyrophosphorylase are intimately intertwined. Plant Physiol 146:289–299. https://doi.org/10.1104/PP.107.109942
Boehlein S, Shaw J, Hannah L, Stewart J (2010) Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase. Plant Physiol 152:85–95. https://doi.org/10.1104/PP.109.146928
Boehlein S, Shaw J, Georgelis N, Hannah L (2014) Enhanced heat stability and kinetic parameters of maize endosperm ADPglucose pyrophosphorylase by alteration of phylogenetically identified amino acids. Arch Biochem Biophys 543:1–9. https://doi.org/10.1016/J.ABB.2013.12.018
Boehlein S, Shaw J, Stewart J, Sullivan B, Hannah L (2015) Enhancing the heat stability and kinetic parameters of the maize endosperm ADP-glucose pyrophosphorylase using iterative saturation mutagenesis. Arch Biochem Biophys 568:28–37. https://doi.org/10.1016/J.ABB.2015.01.008
Bowsher CG, Scrase-Field EFAL, Esposito S, Emes MJ, Tetlow IJ (2007) Characterization of ADP-glucose transport across the cereal endosperm amyloplast envelope. J Exp Bot 58:1321–1332. https://doi.org/10.1093/jxb/erl297
Brown K, Pompeo F, Dixon S, Mengin-Lecreulx D, Cambillau C, Bourne Y (1999) Crystal structure of the bifunctional N-acetylglucosamine 1-phosphate uridyltransferase from Escherichia coli: a paradigm for the related pyrophosphorylase superfamily. EMBO J 18:4096–4107. https://doi.org/10.1093/EMBOJ/18.15.4096
Burton RA, Johnson PE, Beckles DM, Fincher GB, Jenner HL, Naldrett MJ, Denyer K (2002) Characterization of the genes encoding the cytosolic and plastidial forms of ADP-glucose pyrophosphorylase in wheat endosperm. Plant Physiol 130:1464–1475. https://doi.org/10.1104/pp.010363
Cereijo AE, Diez MDA, Ballicora MA, Iglesias AA (2018) Regulatory properties of the ADP-glucose pyrophosphorylase from the clostridial Firmicutes member Ruminococcus albus. J Bacteriol. https://doi.org/10.1128/JB.00172-18
Charng YY, Iglesias AA, Preiss J (1994) Structure-function relationships of cyanobacterial ADP-glucose pyrophosphorylase. Site-directed mutagenesis and chemical modification of the activator-binding sites of ADP-glucose pyrophosphorylase from Anabaena PCC 7120. J Biol Chem 269:24107–24113
Cifuente JO, Comino N, Madariaga-Marcos J, López-Fernández S, García-Alija M, Agirre J, Albesa-Jové D, Guerin ME (2016) Structural basis of glycogen biosynthesis regulation in bacteria. Structure 24:1613–1622. https://doi.org/10.1016/J.STR.2016.06.023
Coutinho P, Deleury E, Davies G, Henrissat B (2003) An evolving hierarchical family classification for glycosyltransferases. J Mol Biol 328:307–317. https://doi.org/10.1016/S0022-2836(03)00307-3
Crevillén P, Ballicora MA, Mérida A, Preiss J, Romero JM (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278:28508–28515. https://doi.org/10.1074/jbc.M304280200
Crevillén P, Ventriglia T, Pinto F, Orea A, Mérida A, Romero JM (2005) Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J Biol Chem 280:8143–8149. https://doi.org/10.1074/jbc.M411713200
Crofts N, Nakamura Y, Fujita N (2017) Critical and speculative review of the roles of multi-protein complexes in starch biosynthesis in cereals. Plant Sci 262:1–8. https://doi.org/10.1016/j.plantsci.2017.05.007
Cupp-Vickery JR, Igarashi RY, Perez M, Poland M, Meyer CR (2008) Structural analysis of ADP-glucose pyrophosphorylase from the bacterium Agrobacterium tumefaciens. Biochemistry 47:4439–4451. https://doi.org/10.1021/BI701933Q
D’Ovidio R, Raiola A, Capodicasa C, Devoto A, Pontiggia D, Roberti S, Galletti R, Conti E, O’Sullivan D, De Lorenzo G (2004) Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins reveals subfunctionalization for defense against fungi and insects. Plant Physiol 135:2424–2435. https://doi.org/10.1104/PP.104.044644
Delvalle D, Dumez S, Wattebled F, Roldan I, Planchot V, Berbezy P, Colonna P, Vyas D, Chatterjee M, Ball S, Merida A, D’Hulst C (2005) Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J 43:398–412. https://doi.org/10.1111/j.1365-313X.2005.02462.x
Derelle E, Ferraz C, Rombauts S, Rouzé P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynié S, Cooke R, Saeys Y, Wuyts J, Jabbari K, Bowler C, Panaud O, Piégu B, Ball SG, Ral J-P, Bouget F-Y, Piganeau G, De Baets B, Picard A, Delseny M, Demaille J, Van de Peer Y, Moreau H (2006) Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA 103:11647–11652. https://doi.org/10.1073/pnas.0604795103
Dong K, Zhen S, Cheng Z, Cao H, Ge P, Yan Y (2015) Proteomic analysis reveals key proteins and phosphoproteins upon seed germination of wheat (Triticum aestivum L.). Front Plant Sci 6:1017. https://doi.org/10.3389/fpls.2015.01017
Ferrero D, Asencion Diez M, Kuhn M, Falaschetti C, Piattoni C, Iglesias A, Ballicora M (2018) On the roles of wheat endosperm ADP-glucose pyrophosphorylase subunits. Front Plant Sci. https://doi.org/10.3389/FPLS.2018.01498
Ferrero DML, Piattoni CV, Asencion Diez MD, Rojas BE, Hartman MD, Ballicora MA, Iglesias AA (2020) Phosphorylation of ADP-glucose pyrophosphorylase during wheat seeds development. Front Plant Sci 11:1058. https://doi.org/10.3389/fpls.2020.01058
Ferretti MV, Hussien RA, Ballicora MA, Iglesias AA, Figueroa CM, Asencion Diez MD (2021) The ADP-glucose pyrophosphorylase from Melainabacteria: a comparative study between photosynthetic and non-photosynthetic bacterial sources. Biochimie. https://doi.org/10.1016/j.biochi.2021.09.011
Figueroa CM, Esper MC, Bertolo A, Demonte AM, Aleanzi M, Iglesias AA, Ballicora MA (2011) Understanding the allosteric trigger for the fructose-1,6-bisphosphate regulation of the ADP-glucose pyrophosphorylase from Escherichia coli. Biochimie 93:1816–1823. https://doi.org/10.1016/j.biochi.2011.06.029
Figueroa CM, Kuhn ML, Falaschetti CA, Solamen L, Olsen KW, Ballicora MA, Iglesias AA (2013) Unraveling the activation mechanism of the potato tuber ADP-glucose pyrophosphorylase. PLoS ONE 8:e66824. https://doi.org/10.1371/JOURNAL.PONE.0066824
Figueroa CM, Piattoni CV, Trípodi KEJ, Podestá FE, Iglesias AA (2016) Carbon photoassimilation and photosynthate partitioning in plants. In: Pessarakli M (ed) Handbook of photosynthesis, 3rd edn. CRC Press, Taylor & Francis Group, Boca Raton, pp 509–535
Figueroa CM, Kuhn ML, Hill BL, Iglesias AA, Ballicora MA (2018) Resurrecting the regulatory properties of the Ostreococcus tauri ADP-glucose pyrophosphorylase large subunit. Front Plant Sci. https://doi.org/10.3389/FPLS.2018.01564
Figueroa CM, Lunn JE, Iglesias AA (2021) Nucleotide-sugar metabolism in plants: the legacy of Luis F. Leloir. J Exp Bot. https://doi.org/10.1093/jxb/erab109
Frueauf JB, Ballicora MA, Preiss J (2002) Alteration of inhibitor selectivity by site-directed mutagenesis of Arg294 in the ADP-glucose pyrophosphorylase from Anabaena PCC 7120. Arch Biochem Biophys 400:208–214. https://doi.org/10.1016/S0003-9861(02)00015-2
Fu Y, Ballicora MA, Leykam JF, Preiss J (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273:25045–25052. https://doi.org/10.1074/jbc.273.39.25045
Georgelis N, Braun EL, Shaw JR, Hannah LC (2007) The two AGPase subunits evolve at different rates in angiosperms, yet they are equally sensitive to activity-altering amino acid changes when expressed in bacteria. Plant Cell 19:1458–1472. https://doi.org/10.1105/tpc.106.049676
Glaring MA, Koch CB, Blennow A (2006) Genotype-specific spatial distribution of starch molecules in the starch granule: a combined CLSM and SEM approach. Biomacromolecules 7:2310–2320. https://doi.org/10.1021/bm060216e
Gomez-Casati DF, Martin M, Busi MV (2013) Polysaccharide-synthesizing glycosyltransferases and carbohydrate binding modules: the case of starch synthase III. Protein Pept Lett 20:856–863
Gómez-Casati DF, Iglesias AA (2002) ADP-glucose pyrophosphorylase from wheat endosperm. Purification and characterization of an enzyme with novel regulatory properties. Planta 214:428–434. https://doi.org/10.1007/S004250100634
Goren A, Ashlock D, Tetlow IJ (2018) Starch formation inside plastids of higher plants. Protoplasma 255:1855–1876. https://doi.org/10.1007/s00709-018-1259-4
Gould SB, Waller RF, McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59:491–517. https://doi.org/10.1146/annurev.arplant.59.032607.092915
Hädrich N, Hendriks JHM, Kötting O, Arrivault S, Feil R, Zeeman SC, Gibon Y, Schulze WX, Stitt M, Lunn JE (2012) Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal starch turnover in Arabidopsis thaliana leaves. Plant J 70:231–242. https://doi.org/10.1111/j.1365-313X.2011.04860.x
Hannah LC, James M (2008) The complexities of starch biosynthesis in cereal endosperms. Curr Opin Biotechnol 19:160–165. https://doi.org/10.1016/j.copbio.2008.02.013
Hedin N, Barchiesi J, Gomez-Casati DF, Iglesias AA, Ballicora MA, Busi MV (2017) Identification and characterization of a novel starch branching enzyme from the picoalgae Ostreococcus tauri. Arch Biochem Biophys. https://doi.org/10.1016/j.abb.2017.02.005
Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133:838–849. https://doi.org/10.1104/pp.103.024513
Hill BL, Wong J, May BM, Huerta FB, Manley TE, Sullivan PRF, Olsen KW, Ballicora MA (2015) Conserved residues of the Pro103–Arg115 loop are involved in triggering the allosteric response of the Escherichia coli ADP-glucose pyrophosphorylase. Protein Sci 24:714–728. https://doi.org/10.1002/pro.2644
Hill BL, Mascarenhas R, Patel H, Asencion Diez M, Wu R, Iglesias AA, Liu D, Ballicora MA (2019) Structural analysis reveals a pyruvate-binding activator site in the Agrobacterium tumefaciens ADP-glucose pyrophosphorylase. J Biol Chem 294:1338–1348. https://doi.org/10.1074/JBC.RA118.004246
Huang B, Hennen-Bierwagen TA, Myers AM (2014) Functions of multiple genes encoding ADP-glucose pyrophosphorylase subunits in maize endosperm, embryo, and leaf. Plant Physiol 164:596–611. https://doi.org/10.1104/pp.113.231605
Hwang SK, Nagai Y, Kim D, Okita TW (2008) Direct appraisal of the potato tuber ADP-glucose pyrophosphorylase large subunit in enzyme function by study of a novel mutant form. J Biol Chem 283:6640–6647. https://doi.org/10.1074/JBC.M707447200
Hwang S-K, Singh S, Maharana J, Kalita S, Tuncel A, Rath T, Panda D, Modi MK, Okita TW (2019) Mechanism underlying heat stability of the rice endosperm cytosolic ADP-glucose pyrophosphorylase. Front Plant Sci 10:70. https://doi.org/10.3389/fpls.2019.00070
Iglesias AA, Barry GF, Meyer C, Bloksberg L, Nakata PA, Greene T, Laughlin MJ, Okita TW, Kishore GM, Preiss J (1993) Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem 268:1081–1086
Iglesias AA, Charng YY, Ball S, Preiss J (1994) Characterization of the kinetic, regulatory, and structural properties of ADP-glucose pyrophosphorylase from Chlamydomonas reinhardtii. Plant Physiol. https://doi.org/10.1104/pp.104.4.1287
Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y (2010) Starch biosynthesis in cereal endosperm. Plant Physiol Biochem 48:383–392. https://doi.org/10.1016/j.plaphy.2010.03.006
Jin X, Ballicora MA, Preiss J, Geiger JH (2005) Crystal structure of potato tuber ADP-glucose pyrophosphorylase. EMBO J 24:694. https://doi.org/10.1038/SJ.EMBOJ.7600551
Kanamaru S, Leiman PG, Kostyuchenko V, Chipman P, Mesyanzhinov V, Arisaka F, Rossmann M (2002) Structure of the cell-puncturing device of bacteriophage T4. Nature 415:553–557. https://doi.org/10.1038/415553A
Kisker C, Schindelin H, Alber BE, Ferry JG, Rees DC (1996) A left-hand beta-helix revealed by the crystal structure of a carbonic anhydrase from the archaeon Methanosarcina thermophila. EMBO J 15:2323
Kleczkowski LA (1999) A phosphoglycerate to inorganic phosphate ratio is the major factor in controlling starch levels in chloroplasts via ADP-glucose pyrophosphorylase regulation. FEBS Lett 448:153–156. https://doi.org/10.1016/S0014-5793(99)00346-4
Kleczkowski LA, Villand P, Luthi E, Olsen OA, Preiss J (1993) Insensitivity of barley endosperm ADP-glucose pyrophosphorylase to 3-phosphoglycerate and orthophosphate regulation. Plant Physiol 101:179–186. https://doi.org/10.1104/PP.101.1.179
Kostrewa D, D’Arcy A, Takacs B, Kamber M (2001) Crystal structures of Streptococcus pneumoniae N-acetylglucosamine-1-phosphate uridyltransferase, GlmU, in apo form at 2.33 A resolution and in complex with UDP-N-acetylglucosamine and Mg(2+) at 1.96 A resolution. J Mol Biol 305:279–289. https://doi.org/10.1006/JMBI.2000.4296
Kuhn ML, Falaschetti CA, Ballicora MA (2009) Ostreococcus tauri ADP-glucose pyrophosphorylase reveals alternative paths for the evolution of subunit roles. J Biol Chem 284:34092–34102. https://doi.org/10.1074/jbc.M109.037614
Kuhn ML, Figueroa CM, Iglesias AA, Ballicora MA (2013) The ancestral activation promiscuity of ADP-glucose pyrophosphorylases from oxygenic photosynthetic organisms. BMC Evol Biol 13:51. https://doi.org/10.1186/1471-2148-13-51
Lee S-K, Hwang S-K, Han M, Eom J-S, Kang H-G, Han Y, Choi S-B, Cho M-H, Bhoo SH, An G, Hahn T-R, Okita TW, Jeon J-S (2007) Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol Biol 65:531–546. https://doi.org/10.1007/s11103-007-9153-z
Leloir LF (1971) Two decades of research on the biosynthesis of saccharides. Science 172:1299–1303. https://doi.org/10.1126/science.172.3990.1299
Lepistö A, Pakula E, Toivola J, Krieger-Liszkay A, Vignols F, Rintamäki E (2013) Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. J Exp Bot 64:3843–3854. https://doi.org/10.1093/jxb/ert216
Linebarger CRL, Boehlein SK, Sewell AK, Shaw J, Hannah LC (2005) Heat stability of maize endosperm ADP-glucose pyrophosphorylase is enhanced by insertion of a cysteine in the N terminus of the small subunit. Plant Physiol 139:1625–1634. https://doi.org/10.1104/pp.105.067637
Lombard V, Golaconda Ramulu H, Drula E, Coutinho P, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. https://doi.org/10.1093/NAR/GKT1178
Lunn JE, Feil R, Hendriks JH, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397:139–148. https://doi.org/10.1042/bj20060083
Lynch M (2006) The origins of eukaryotic gene structure. Mol Biol Evol 23:450–468. https://doi.org/10.1093/MOLBEV/MSJ050
Machtey M, Kuhn ML, Flasch DA, Aleanzi M, Ballicora MA, Iglesias AA (2012) Insights into glycogen metabolism in chemolithoautotrophic bacteria from distinctive kinetic and regulatory properties of ADP-glucose pyrophosphorylase from Nitrosomonas europaea. J Bacteriol. https://doi.org/10.1128/JB.00810-12
MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ (2017) Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot 68:4433–4453. https://doi.org/10.1093/jxb/erx291
Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P (2009) NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA 106:9908–9913. https://doi.org/10.1073/pnas.0903559106
Mugford ST, Fernandez O, Brinton J, Flis A, Krohn N, Encke B, Feil R, Sulpice R, Lunn JE, Stitt M, Smith AM (2014) Regulatory properties of ADP glucose pyrophosphorylase are required for adjustment of leaf starch synthesis in different photoperiods. Plant Physiol 166:1733–1747. https://doi.org/10.1104/pp.114.247759
Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M, Ishihama Y, Shirasu K (2010) Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol 153:1161–1174. https://doi.org/10.1104/pp.110.157347
Olsen L, Roderick S (2001) Structure of the Escherichia coli GlmU pyrophosphorylase and acetyltransferase active sites. Biochemistry 40:1913–1921. https://doi.org/10.1021/BI002503N
Pandey MK, Rani NS, Madhav MS, Sundaram RM, Varaprasad GS, Sivaranjani AK, Bohra A, Kumar GR, Kumar A (2012) Different isoforms of starch-synthesizing enzymes controlling amylose and amylopectin content in rice (Oryza sativa L.). Biotechnol Adv 30:1697–1706. https://doi.org/10.1016/j.biotechadv.2012.08.011
Patron NJ, Greber B, Fahy BF, Laurie DA, Parker ML, Denyer K (2004) The lys5 mutations of barley reveal the nature and importance of plastidial ADP-Glc transporters for starch synthesis in cereal endosperm. Plant Physiol 135:2088–2097. https://doi.org/10.1104/pp.104.045203
Petreikov M, Eisenstein M, Yeselson Y, Preiss J, Schaffer AA (2010) Characterization of the AGPase large subunit isoforms from tomato indicates that the recombinant L3 subunit is active as a monomer. Biochem J 428:201–212. https://doi.org/10.1042/BJ20091777
Raetz CRH, Roderick SL (1995) A left-handed parallel β helix in the structure of UDP-N-acetylglucosamine acyltransferase. Science 270:997–1000. https://doi.org/10.1126/SCIENCE.270.5238.997
Raguin A, Ebenhoh O (2017) Design starch: stochastic modeling of starch granule biogenesis. Biochem Soc Trans 45:885–893. https://doi.org/10.1042/BST20160407
Ral JP, Derelle E, Ferraz C, Wattebled F, Farinas B, Corellou F, Buleon A, Slomianny MC, Delvalle D, d’Hulst C, Rombauts S, Moreau H, Ball S (2004) Starch division and partitioning. A mechanism for granule propagation and maintenance in the picophytoplanktonic green alga Ostreococcus tauri. Plant Physiol 136:3333–3340. https://doi.org/10.1104/pp.104.044131
Rose CM, Venkateshwaran M, Volkening JD, Grimsrud PA, Maeda J, Bailey DJ, Park K, Howes-Podoll M, den Os D, Yeun LH, Westphall MS, Sussman MR, Ané J-M, Coon JJ (2012) Rapid phosphoproteomic and transcriptomic changes in the rhizobia-legume symbiosis. Mol Cell Proteomics 11:724–744. https://doi.org/10.1074/mcp.M112.019208
Rossman MG, Liljas A, Brändén CI, Banaszak LJ (1975) 2 Evolutionary and structural relationships among dehydrogenases. Enzymes 11:61–102. https://doi.org/10.1016/S1874-6047(08)60210-3
Rösti S, Denyer K (2007) Two paralogous genes encoding small subunits of ADP-glucose pyrophosphorylase in maize, Bt2 and L2, replace the single alternatively spliced gene found in other cereal species. J Mol Evol 65:316–327. https://doi.org/10.1007/s00239-007-9013-0
Rösti S, Rudi H, Rudi K, Opsahl-Sorteberg H-G, Fahy B, Denyer K (2006) The gene encoding the cytosolic small subunit of ADP-glucose pyrophosphorylase in barley endosperm also encodes the major plastidial small subunit in the leaves. J Exp Bot 57:3619–3626. https://doi.org/10.1093/jxb/erl110
Seferoglu AB, Koper K, Can FB, Cevahir G, Kavakli IH (2014) Enhanced heterotetrameric assembly of potato ADP-glucose pyrophosphorylase using reverse genetics. Plant Cell Physiol 55:1473–1483. https://doi.org/10.1093/PCP/PCU078
Seferoglu AB, Gul S, Dikbas UM, Baris I, Koper K, Caliskan M, Cevahir G, Kavakli IH (2016) Glu-370 in the large subunit influences the substrate binding, allosteric, and heat stability properties of potato ADP-glucose pyrophosphorylase. Plant Sci 252:125–132. https://doi.org/10.1016/J.PLANTSCI.2016.07.007
Seung D, Boudet J, Monroe J, Schreier TB, David LC, Abt M, Lu KJ, Zanella M, Zeeman SC (2017) Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 29:1657–1677. https://doi.org/10.1105/tpc.17.00222
Seung D, Schreier TB, Burgy L, Eicke S, Zeeman SC (2018) Two plastidial coiled-coil proteins are essential for normal starch granule initiation in Arabidopsis. Plant Cell 30:1523–1542. https://doi.org/10.1105/tpc.18.00219
Shannon JC, Pien FM, Cao H, Liu KC (1998) Brittle-1, an adenylate translocator, facilitates transfer of extraplastidial synthesized ADP-glucose into amyloplasts of maize endosperms. Plant Physiol 117:1235–1252. https://doi.org/10.1104/pp.117.4.1235
Sheng J, Preiss J (1997) Arginine294 is essential for the inhibition of Anabaena PCC 7120 ADP-glucose pyrophosphorylase by phosphate. Biochemistry 36:13077–13084. https://doi.org/10.1021/bi9713355
Sivaraman J, Sauvé V, Matte A, Cygler M (2002) Crystal structure of Escherichia coli glucose-1-phosphate thymidylyltransferase (RffH) complexed with dTTP and Mg2+*. J Biol Chem 277:44214–44219. https://doi.org/10.1074/JBC.M206932200
Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism—more than the icing on the cake. Plant J 61:1067–1091. https://doi.org/10.1111/j.1365-313X.2010.04142.x
Sulzenbacher G, Gal L, Peneff C, Fassy F, Bourne Y (2001) Crystal structure of Streptococcus pneumoniae N-acetylglucosamine-1-phosphate uridyltransferase bound to acetyl-coenzyme A reveals a novel active site architecture. J Biol Chem 276:11844–11851. https://doi.org/10.1074/JBC.M011225200
Suzuki E, Suzuki R (2013) Variation of storage polysaccharides in phototrophic microorganisms. J Appl Glycosci 60:21–27. https://doi.org/10.5458/jag.jag.JAG-2012_016
Takata H, Takaha T, Okada S, Takagi M, Imanaka T (1997) Characterization of a gene cluster for glycogen biosynthesis and a heterotetrameric ADP-glucose pyrophosphorylase from Bacillus stearothermophilus. J Bacteriol 179:4689–4698. https://doi.org/10.1128/jb.179.15.4689-4698.1997
Tetlow IJ, Davies EJ, Vardy KA, Bowsher CG, Burrell MM, Emes MJ (2003) Subcellular localization of ADPglucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform. J Exp Bot 54:715–725. https://doi.org/10.1093/jxb/erg088
Thormählen I, Ruber J, von Roepenack-Lahaye E, Ehrlich S-M, Massot V, Hümmer C, Tezycka J, Issakidis-Bourguet E, Geigenberger P (2013) Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant Cell Environ 36:16–29. https://doi.org/10.1111/j.1365-3040.2012.02549.x
Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14:2191–2213. https://doi.org/10.1105/tpc.003640
Tocchini-Valentini GD, Fruscoloni P, Tocchini-Valentini GP (2005) Structure, function, and evolution of the tRNA endonucleases of Archaea: an example of subfunctionalization. Proc Natl Acad Sci USA 102:8933–8938. https://doi.org/10.1073/PNAS.0502350102
Tuncel A, Okita TW (2013) Improving starch yield in cereals by over-expression of ADPglucose pyrophosphorylase: expectations and unanticipated outcomes. Plant Sci 211:52–60. https://doi.org/10.1016/j.plantsci.2013.06.009
Tuncel A, Cakir B, Hwang S-K, Okita TW (2014) The role of the large subunit in redox regulation of the rice endosperm ADP-glucose pyrophosphorylase. FEBS J 281:4951–4963. https://doi.org/10.1111/febs.13041
Vandromme C, Spriet C, Dauvillee D, Courseaux A, Putaux JL, Wychowski A, Krzewinski F, Facon M, D’Hulst C, Wattebled F (2019) PII1: a protein involved in starch initiation that determines granule number and size in Arabidopsis chloroplast. New Phytol 221:356–370. https://doi.org/10.1111/nph.15356
Ventriglia T, Kuhn ML, Ruiz MT, Ribeiro-Pedro M, Valverde F, Ballicora MA, Preiss J, Romero JM (2008) Two Arabidopsis ADP-glucose pyrophosphorylase large subunits (APL1 and APL2) are catalytic. Plant Physiol 148:65–76. https://doi.org/10.1104/pp.108.122846
Wilkens C, Svensson B, Moller MS (2018) Functional roles of starch binding domains and surface binding sites in enzymes involved in starch biosynthesis. Front Plant Sci 9:1652. https://doi.org/10.3389/fpls.2018.01652
Yu G, Lv Y, Shen L, Wang Y, Qing Y, Wu N, Li Y, Huang H, Zhang N, Liu Y, Hu Y, Liu H, Zhang J, Huang Y (2019) The proteomic analysis of maize endosperm protein enriched by Phos-tagtm reveals the phosphorylation of brittle-2 subunit of ADP-Glc pyrophosphorylase in starch biosynthesis process. Int J Mol Sci. https://doi.org/10.3390/ijms20040986
Zhu G-R, Yan X, Zhu D, Deng X, Wu J-S, Xia J, Yan Y-M (2018) Lysine acetylproteome profiling under water deficit reveals key acetylated proteins involved in wheat grain development and starch biosynthesis. J Proteomics 185:8–24. https://doi.org/10.1016/j.jprot.2018.06.019
Acknowledgements
CMF, MDAD and AAI are Researchers from CONICET. We thank Jaina Bhayani for feedback on the writing of the manuscript.
Funding
This work was supported by grants from Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (PICT-2017-1515 and PICT-2018-00929 to AAI, PICT-2018-00698 to MDAD and PICT-2018-00865 to CMF), Universidad Nacional del Litoral (CAI+D 2020 to CMF), CONICET (PUE-2016-0040 to IAL), the Max Planck Society (Partner Group for Plant Biochemistry to CMF) and the National Science Foundation (MCB 1616851 to MAB).
Author information
Authors and Affiliations
Contributions
Conceptualization: all authors; Writing—original draft preparation: all authors; Writing—review and editing: all authors; Funding acquisition: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueroa, C.M., Asencion Diez, M.D., Ballicora, M.A. et al. Structure, function, and evolution of plant ADP-glucose pyrophosphorylase. Plant Mol Biol 108, 307–323 (2022). https://doi.org/10.1007/s11103-021-01235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01235-8