Abstract
Background
Inhalational corticosteroids (ICS) were observed to increase the pneumonia risk in chronic obstructive pulmonary airway disorder (COPD). However, it is unknown whether any differences exist between the drugs within the ICS class.
Aim
This study aimed to evaluate the risk of pneumonia associated with different ICS and identify factors that predict pneumonia in patients with moderate-to-severe COPD using a network meta-analysis.
Method
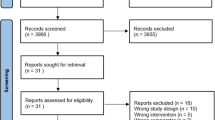
Electronic databases (Medline, Cochrane CENTRAL and Google Scholar) were searched for trials comparing ICS in COPD patients. The outcomes were pneumonia and serious pneumonia. Odds ratios (OR) with 95% confidence interval (95% CI) were estimated. Meta-regression was used to identify the predictors. The strength of evidence was graded using the Grading of Recommendations, Assessment, Development, and Evaluations approach.
Results
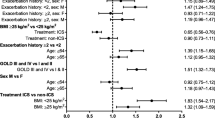
Sixty-six studies (103,347 participants) were included. Fluticasone (OR: 1.46; 95% CI: 1.26, 1.7), mometasone (OR: 2.2; 95% CI: 1.05, 4.6), and beclometasone (OR: 1.7; 95% CI: 1.1, 2.6) were observed with an increased pneumonia risk compared to placebo. Fluticasone (OR: 1.5; 95% CI: 1.3, 1.7) was observed with an increased risk of serious pneumonia. High doses (OR: 1.2; 95% CI: 1.03, 1.4), BMI ≥ 25 kg/m2 (OR: 1.6; 95% CI: 1.1, 2.2), and history of exacerbations in the preceding year predicted the pneumonia risk. Evidence strength was moderate.
Conclusion
ICS class differences in pneumonia risk were observed in terms of pooled effect estimates but it is unlikely that any clinically relevant differences exist. Risk–benefit analysis supports ICS use in moderate-severe COPD.




Similar content being viewed by others
References
Park SC, Kim DW, Park EC, et al. Mortality of patients with chronic obstructive pulmonary disease: a nationwide population based cohort study. Korean J Intern Med. 2019;34(6):1272–8.
Global Initiative for Asthma 2022. Global strategy for asthma management and prevention. Available at: https://ginasthma.org/wp-content/uploads/2022/05/GINA-Main-Report-2022-FINAL-22-05-03-WMS.pdf. Accessed 9 Nov 2023.
Miravitlles M, Roman-Rodríguez M, Ribera X, et al. Inhaled corticosteroid use among COPD patients in primary care in Spain. Int J Chronic Obstr Pulm Dis. 2022;17:245–58.
Quint JK, Ariel A, Barnes PJ. Rational use of inhaled corticosteroids for the treatment of COPD. NPJ Prim Care Respir Med. 2023;33(1):27.
GOLD 2023 guidelines. Available at: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf. Accessed 12 March 2024.
Celli BR, Anderson JA, Cowans NJ, et al. Pharmacotherapy and lung function decline in patients with chronic obstructive pulmonary disease: a systematic review. Am J Respir Crit Care Med. 2021;203:689-98. https://doi.org/10.1164/rccm.202005-1854OC.
Calverley P. Reigniting the TORCH: chronic obstructive pulmonary disease mortality and inhaled corticosteroids revisited. Am J Respir Crit Care Med. 2021;203(5):531–2.
Cates C. Inhaled corticosteroids in COPD: quantifying risks and benefits. Thorax. 2013;68(6):499–500.
Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. Open Respir Med J. 2014;8:59–65.
Mkorombindo T, Dransfield MT. Inhaled corticosteroids in chronic obstructive pulmonary disease: benefits and risks. Clin Chest Med. 2020;41(3):475–84.
Zhang Q, Li S, Zhou W, et al. Risk of pneumonia with different inhaled corticosteroids in COPD patients: a meta-analysis. COPD. 2020;17(4):462–9.
Yang M, Du Y, Chen H, et al. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019;77: 105950.
Suissa S. Inhaled corticosteroids and pneumonia mortality in COPD patients. Eur Respir J. 2019;54(3):1901276.
Chen H, Sun J, Huang Q, et al. Inhaled corticosteroids and the pneumonia risk in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12: 691621.
Lodise TP, Li J, Gandhi HN, et al. Intraclass difference in pneumonia risk with fluticasone and budesonide in COPD: a systematic review of evidence from direct-comparison studies. Int J Chronic Obstr Pulm Dis. 2020;15:2889–900.
Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(9):1906–16.
Proposal for pneumonia risk with ICS. Available at: https://osf.io/tegpk. Accessed 1 Jan 2024.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Gjerdevik M, Heuch I. Improving the error rates of the Begg and Mazumdar test for publication bias in fixed effects meta-analysis. BMC Med Res Methodol. 2014;14:109.
Asthma: diagnosis, monitoring and chronic asthma management. Available at: https://www.nice.org.uk/guidance/ng80/resources/asthma-diagnosis-monitoring-and-chronic-asthma-management-pdf-1837687975621. Accessed 2 Nov 2023.
EpiGear International. Available at: https://www.epigear.com/index.html. Accessed 6 Nov 2023.
GRADE handbook. Introduction to GRADE handbook. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 6 Nov 2023.
VOSviewer version 1.6.20. Available at: https://www.vosviewer.com/. Accessed 4 Nov 2023.
Litmaps (2023). Available at: https://www.litmaps.com/. Accessed 4 Nov 2023.
Wallace BC, Lajeunesse MJ, Schmid CH, et al. OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol Evol. 2017;8(8):941–7.
Thorlund K, Engstrom J, Wetterslev J, et al. Trial sequential analysis. Copenhagen trial unit. Available at: https://ctu.dk/tsa/. Accessed 4 Nov 2023.
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone–salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007;146:45-55.
Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD J Chronic Obstr Pulm Dis. 2009;6(5):320–9.
Bansal S, Anderson M, Anzueto A, et al. Single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy versus tiotropium monotherapy in patients with COPD. Prim Care Respir Med. 2021;31(1):29.
Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study). Int J Chronic Obstr Pulm Dis. 2016;4(11):193–205.
Beeh KM, Kuna P, Corradi M, et al. Comparison of Dry-powder inhaler and pressurized metered-dose inhaler formulations of extrafine beclomethasone dipropionate/formoterol fumarate/glycopyrronium in patients with COPD: the TRI-D randomized controlled trial. COPD. 2021;16:79–89.
Betsuyaku T, Kato M, Fujimoto K, et al. A randomized trial of symptom-based management in Japanese patients with COPD. COPD. 2018;13:2409–23.
Bhatt S, Dransfield M, Cockcroft J, et al. A randomized trial of once-daily fluticasone furoate/vilanterol or vilanterol versus placebo to determine effects on arterial stiffness in COPD. COPD. 2017;12:351–65.
Burge PS, Calverley PM, Jones PW et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–303.
Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–9.
Calverley PM, Rennard S, Nelson HS, et al. One-year treatment with mometasone furoate in chronic obstructive pulmonary disease. Respir Res. 2008;9(1):73.
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89.
Calverley PMA, Kuna P, Monsó E, et al. Beclomethasone/formoterol in the management of COPD: A randomised controlled trial. Respir Med. 2010;104(12):1858–68.
Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–39.
Cheng SL, Su KC, Wang HC, et al. Chronic obstructive pulmonary disease treated with inhaled medium- or high-dose corticosteroids: a prospective and randomized study focusing on clinical efficacy and the risk of pneumonia. Drug Des. 2014:601-7.
Covelli H, Pek B, Schenkenberger I, et al. Efficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular risk. COPD. 2015;1.
Doherty D, Tashkin D, Kerwin, et al. Effects of mometasone furoate/formoterol fumarate fixed-dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52-week Phase III trial in subjects with moderate-to-very severe COPD. COPD. 2012;57.
Donohue JF, Worsley S, Zhu CQ, et al. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109(7):870–81.
Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–23.
Dransfield MT, Cockcroft JR, Townsend RR, et al. Effect of fluticasone propionate/salmeterol on arterial stiffness in patients with COPD. Respir Med. 2011;105(9):1322–30.
Dransfield MT, Crim C, Criner GJ, et al. Risk of exacerbation and pneumonia with single-inhaler triple versus dual therapy in IMPACT. Annals ATS. 2021;18(5):788–98.
Fatima S, Tariq F, Saqib Ur Rehman M, et al. Risk of pneumonia with inhaled corticosteroid/long-acting β2 agonist therapy in chronic obstructive pulmonary disease. PJMHS. 2023;17(4):12–6.
Ferguson GT, Anzueto A, Fei R, et al. Effect of fluticasone propionate/salmeterol (250/50 μg) or salmeterol (50 μg) on COPD exacerbations. Respir Med. 2008;102(8):1099–108.
Ferguson GT, Brown N, Compton C, et al. Once-daily single-inhaler versus twice-daily multiple-inhaler triple therapy in patients with COPD: lung function and health status results from two replicate randomized controlled trials. Respir Res. 2020;21(1):131.
Ferguson GT, Papi A, Anzueto A, et al. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. Eur Respir J. 2018;52(3):1801334.
Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–58.
Ferguson GT, Tashkin DP, Skärby T, et al. Effect of budesonide/formoterol pressurized metered-dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6-month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) study. Respir Med. 2017;132:31–41.
Frith PA, Ashmawi S, Krishnamurthy S, et al. Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non-frequently exacerbating COPD patients: the FLASH randomized controlled trial. Respirology. 2018;23(12):1152–9.
Fukuchi Y, Samoro R, Fassakhov R, et al. Budesonide/formoterol via Turbuhaler® versus formoterol via T urbuhaler® in patients with moderate to severe chronic obstructive pulmonary disease: phase III multinational study results. Respirology. 2013;18(5):866–73.
Hanania NA, Crater GD, Morris AN, et al. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91–101.
Hanania NA, Papi A, Anzueto A, et al. Efficacy and safety of two doses of budesonide/formoterol fumarate metered dose inhaler in COPD. ERJ Open Res. 2020;6(2):00187–2019.
Huang K, Guo Y, Kang J, et al. The efficacy of adding budesonide/formoterol to ipratropium plus theophylline in managing severe chronic obstructive pulmonary disease: an open-label, randomized study in China. Ther Adv Respir Dis. 2019;13:175346661985350.
Ichinose M, Fukushima Y, Inoue Y, et al. Long-term safety and efficacy of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler formulated using co-suspension delivery technology in Japanese patients with COPD. COPD. 2019;14:2993–3002.
Jung KS, Park HY, Park SY et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir med. 2012;106:382-9.
Kardos P, Wencker M, Glaab T, et al. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(2):144–9.
Kerwin EM, Ferguson GT, Mo M, et al. Bone and ocular safety of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a 52-week randomized study. Respir Res. 2019;20(1):167.
Kerwin EM, Scott-Wilson C, Sanford L, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPD. Respir Med. 2013;107(4):560–9.
Lee S, Xie C, Yunus F, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East Asia. Respirology. 2016;21(1):119–27.
Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–80.
Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–94.
Martinez FJ, Boscia J, Feldman G, et al. Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trial. Respir Med. 2013;107(4):550–9.
Muiser S, Imkamp K, Seigers D, et al. Budesonide/formoterol maintenance and reliever therapy versus fluticasone/salmeterol fixed-dose treatment in patients with COPD. Thorax. 2023;78(5):451–8.
Ohar JA, Crater GD, Emmett A, et al. Fluticasone propionate/salmeterol 250/50 μg versus salmeterol 50 μg after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15(1):105.
Panettieri RA Jr, Camargo CA Jr, Cheema T, et al. Effect of recent exacerbation history on the efficacy of once-daily single-inhaler fluticasone furoate/umeclidinium/vilanterol triple therapy in patients with chronic obstructive pulmonary disease in the FULFIL trial. Int J Chronic Obstr Pulm Dis. 2022;1(17):2043–52.
Papi A, Dokic D, Tzimas W, et al. Fluticasone propionate/formoterol for COPD management: a randomized controlled trial. COPD. 2017;12:1961–71.
Pepin JL, Cockcroft JR, Midwinter D, et al. Long-acting bronchodilators and arterial stiffness in patients with COPD. Chest. 2014;146(6):1521–30.
Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48.
Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–65.
Rossi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44:1548-56.
Sharafkhaneh A, Southard JG, Goldman M, et al. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–68.
Siler TM, Nagai A, Scott-Wilson CA, et al. A randomised, phase III trial of once-daily fluticasone furoate/vilanterol 100/25 μg versus once-daily vilanterol 25 μg to evaluate the contribution on lung function of fluticasone furoate in the combination in patients with COPD. Respir Med. 2017;123:8–17.
Singh D, Worsley S, Zhu CQ, et al. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med. 2015;15(1):91.
Tashkin DP, Rennard SI, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000.
Tashkin DP, Doherty DE, Kerwin E, et al. Efficacy and safety of a fixed-dose combination of mometasone furoate and formoterol fumarate in subjects with moderate to very severe COPD: results from a 52-week Phase III trial. Int J Chronic Obstr Pulm Dis. 2012;7:43–55.
Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. The Lancet. 2016;387(10030):1817–26.
Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–60.
Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. The Lancet. 2017;389(10082):1919–29.
Vestbo J, Søorensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. The Lancet. 1999;353(9167):1819–23.
Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48(4):1030–9.
Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60.
Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34.
Wedzicha JA, Calverley PMA, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.
Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108(8):1153–62.
Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–50.
Zheng J, Baldi S, Zhao L, et al. Efficacy and safety of single-inhaler extrafine triple therapy versus inhaled corticosteroid plus long-acting beta2 agonist in eastern Asian patients with COPD: the TRIVERSYTI randomised controlled trial. Respir Res. 2021;22(1):90.
Zheng J, De Guia T, Wang-Jairaj J, et al. Efficacy and safety of fluticasone furoate/vilanterol (50/25 mcg; 100/25 mcg; 200/25 mcg) in Asian patients with chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Curr Med Res Opin. 2015;31(6):1191–200.
Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. COPD. 2015;10:1015–26.
EMA completes review of inhaled corticosteroids for chronic obstructive pulmonary disease. Available at: https://www.ema.europa.eu/en/news/ema-completes-review-inhaled-corticosteroids-chronic-obstructive-pulmonary-disease. Accessed 5 Nov 2023
Choi JH, Jeong KB, Park YH, et al. Comparison of risk of pneumonia caused by fluticasone propionate versus budesonide in chronic obstructive pulmonary disease: a nationwide retrospective cohort study. Int J Chronic Obstr Pulm Dis. 2021;16:3229–37.
Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ. 2013;346: f3306.
Maassen van den Brink KI, Boorsma M, Staal-van den Brekel AJ, et al. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol. 2008;66(1):27–35.
Dalby C, Polanowski T, Larsson T, et al. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10(1):104.
Yebyo HG, Braun J, Menges D, et al. Personalising add-on treatment with inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a benefit-harm modelling study. Lancet Digit Health. 2021;3(10):e644–53.
Sibila O, Soto-Gomez N, Restrepo MI. The risk and outcomes of pneumonia in patients on inhaled corticosteroids. Pulm Pharmacol Ther. 2015;32:130–6.
Kim RY, Glick C, Furmanek S, et al. Association between body mass index and mortality in hospitalised patients with community-acquired pneumonia. ERJ Open Res. 2021;7(1):00736–2020.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sridharan, K., Sivaramakrishnan, G. Intraclass comparison of inhaled corticosteroids for the risk of pneumonia in chronic obstructive pulmonary airway disorder: a network meta-analysis and meta-regression. Int J Clin Pharm (2024). https://doi.org/10.1007/s11096-024-01736-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11096-024-01736-8