Abstract
Background
There have been cases reporting anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) and associated serious gastrointestinal (GI) adverse drug reactions (gastrointestinal obstruction, perforation, and ulceration). These adverse drug reactions are not in the drug package inserts, and the drug relationships are not proven in the literature.
Aim
We aimed to examine the potential association between GI obstruction, perforation, and ulceration, and ALK-TKIs by data mining of the US FDA Adverse Event Reporting System (FAERS).
Method
We conducted a disproportionality analysis of GI obstruction, perforation, and ulceration by estimating the reporting odds ratios (ROR) and the information component (IC) with 95% confidence intervals.
Results
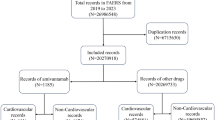
A total of 279 cases of ALK-TKI-associated GI obstruction, perforation, and ulceration from January 1, 2011, to December 31, 2020, were identified. GI obstruction, perforation, and ulceration cause 16% of cases of death. A significantly increased reporting rate for GI obstruction [ROR 1.77 (1.45–2.15); IC 0.82 (0.53–2.03)] and perforation [ROR 1.61 (1.28–2.02); IC 0.68 (0.35–1.92)] was observed for ALK-TKIs as a drug class. The signal of GI ulceration was detected only in crizotinib [ROR 1.23 (1.01–1.50); IC 0.29 (0.01–1.51)]. A statistically significant ROR and IC emerged for the site of the esophagus.
Conclusion
Overall, the pharmacovigilance study of the FAERS indicates slightly increased reporting of GI obstruction and perforation, which may cause severe or even fatal outcomes among ALK-TKIs users.


Similar content being viewed by others
References
Barreca A, Lasorsa E, Riera L, et al. Anaplastic lymphoma kinase in human cancer. J Mol Endocrinol. 2011;47(1):R11–23.
Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31(8):1105–11.
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Khan M, Lin J, Liao G, et al. ALK Inhibitors in the treatment of ALK positive NSCLC. Front Oncol. 2018;8:557.
Wang L, Wang W. Safety and efficacy of anaplastic lymphoma kinase tyrosine kinase inhibitors in non-small cell lung cancer. Oncol Rep. 2021.
Zhu Q, Hu H, Weng DS, et al. Pooled safety analyses of ALK-TKI inhibitor in ALK-positive NSCLC. BMC Cancer. 2017;17(1):412.
Schaefer ES, Baik C. Proactive management strategies for potential gastrointestinal adverse reactions with ceritinib in patients with advanced ALK-positive non-small-cell lung cancer. Cancer Manag Res. 2016;8:33–8.
Yanagisawa A, Hayama N, Amano H, et al. Crizotinib-induced rectal perforation with abscess. Internal Med. 2017;56(23):3211–3.
Sussman TA, Khunger M, Velcheti V. A case of crizotinib-induced esophageal ulcers. Lung Cancer Manag. 2017;6(1):5–7.
Jalil AA, Craig J, Bajaj R, et al. Severe ulcerative esophagitis induced by Crizotinib therapy. ACG Case Rep J. 2014;1(2):82–4.
Duan R, Zhang X, Du J, et al. Post-marketing drug safety evaluation using data mining based on FAERS. In: Data mining and big data: second international conference, DMBD 2017, Fukuoka, Japan, July 27–August 1, 2017 Proceedings DMBD (conference) (2nd : 2017: Fukuoka, Japan); 2017. pp. 379–89.
Meng L, Huang J, Jia Y, et al. Assessing fluoroquinolone-associated aortic aneurysm and dissection: Data mining of the public version of the FDA adverse event reporting system. Int J Clin Pract. 2019;73(5):e13331.
Meng L, Yang B, Qiu F, et al. Lung Cancer adverse events reports for angiotensin-converting enzyme inhibitors: data mining of the FDA adverse event reporting system database. Front Med. 2021;8:594043.
Böhm R, von Hehn L, Herdegen T, et al. OpenVigil FDA–inspection of US American adverse drug events pharmacovigilance data and novel clinical applications. PloS One. 2016;11(6).
Böhm R, Höcker J, Cascorbi I, et al. OpenVigil—free eyeballs on AERS pharmacovigilance data. Nat Biotechnol. 2012;30(2):137.
Jaasu NM, Kamaraj R, Seetharaman R. MedDRA (Medical Dictionary for Regulatory Activities). Res J Pharm Technol. 2018;11(10):4751–4.
Meng L, Yang B, Qiu F, et al. Lung cancer adverse events reports for angiotensin-converting enzyme inhibitors: data mining of the FDA Adverse event reporting system database. Front Med. 2021;8(36).
Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2010;18(6):427–36.
Bate A, Pariente A, Hauben M, et al. Quantitative signal detection and analysis in pharmacovigilance. Mann’s Pharmacovigil. 2014;331–54.
Harpaz R, DuMouchel W, LePendu P, et al. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93(6):539–46.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.
Ehrenstein V, Huang K, Kahlert J, et al. Outcomes in patients with lung cancer treated with crizotinib and erlotinib in routine clinical practice: a post-authorization safety cohort study conducted in Europe and in the United States. Pharmacoepidemiol Drug Saf. 2021;30(6):758–69.
Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II Global study. J Clin Oncol. 2016;34(7):661–8.
Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592–8.
Park J, Yoshida K, Kondo C, et al. Crizotinib-induced esophageal ulceration: a novel adverse event of crizotinib. Lung Cancer. 2013;81(3):495–6.
Sawada T, Maeno K, Joh T. Esophageal ulcer in a lung cancer patient. Crizotinib-induced esophageal injury. Gastroenterology. 2015;149(2):e6-7.
Schmassmann A, Stettler C, Poulsom R, et al. Roles of hepatocyte growth factor and its receptor Met during gastric ulcer healing in rats. Gastroenterology. 1997;113(6):1858–72.
Baatar D, Jones MK, Pai R, et al. Selective cyclooxygenase-2 blocker delays healing of esophageal ulcers in rats and inhibits ulceration-triggered c-Met/hepatocyte growth factor receptor induction and extracellular signal-regulated kinase 2 activation. Am J Pathol. 2002;160(3):963–72.
Michel C, Scosyrev E, Petrin M, et al. Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles? Clin Drug Investig. 2017;37(5):415.
Raschi E, Gatti M, Gelsomino F, et al. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from pharmacovigilance. Target Oncol. 2020;1–18.
Acknowledgements
None.
Funding
This research was funded by Chongqing Health Commission (2020FYYX058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, J., Zhao, Y., Cao, Y. et al. Anaplastic lymphoma kinase tyrosine kinase inhibitors associated gastrointestinal obstruction, perforation, and ulceration: an analysis of the FDA adverse event reporting system database (FAERS). Int J Clin Pharm 44, 993–1003 (2022). https://doi.org/10.1007/s11096-022-01425-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01425-4