Abstract
Osimertinib was a third-generation, irreversible epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), which approved by the US Food and Drug Administration (FDA) in 2015 for treatment of non-small cell lung cancer (NSCLC). Our study was to explore the adverse events (AEs) caused by osimertinib through data mining of the US FDA Adverse Event Reporting System (FAERS), and provide reference for clinical safety. Data of osimertinib were collected from the FAERS database covering the period from first quarter of 2016 to the fourth quarter of 2021. Disproportionality analyses was employed to quantify the associated AE signals of osimertinib and detect the risk signals from the data in the FAERS database. Reporting odds ratio (ROR) was used to detect the risk signals from the data in the FAERS database. The definition relied on system organ class (SOCs) and preferred terms (PTs) by the Medical Dictionary for Regulatory Activities (MedDRA). Totally, 9,704,33 reports were collected from the FAERS database, 10,804 reports of osimertinib were identified as the ‘primary suspected (PS)’ AEs. Osimertinib induced AEs occurred in 27 organ systems. 68 significant disproportionality PTs satisfying with the four algorithms were retained at the same time. Unexpected significant AEs such as scrotal volvulus, hepatic function abnormal, venous thromboembolisms might also occur. The median onset time of osimertinib-associated AEs was 58 days (interquartile range [IQR] 14–212 days), and the majority of the AEs occurred within the first 30 days after osimertinib initiation. Our study found significant new AEs signals of osimertinib and might provide support for clinical monitoring and risk identification of osimertinib.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death in the world currently, accounting for about 80–85% of all lung cancers1,2. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), including gefitinib, erlotinib, and afatinib, are the first-line treatments for NSCLC patients harboring EGFR mutations3. However, up to 50% of the patients treated with first-line EGFR-TKIs acquire resistance to these drugs, and acquisition of the T790M mutation is the main mechanism responsible for the progress of resistance4. Osimertinib is a third-generation EGFR-TKI, which is suitable for patients with EGFR T790M mutation positive NSCLC whose disease progresses on or after EGFR-TKI therapy5. Osimertinib was approved by U.S. Food and Drug Administration (FDA) in November 2015, later in European, Japan and China in 2017. Preclinical studies and phase 1 clinical data from the AURA trial suggested that osimertinib might also be an effective first-line therapy for patients with EGFR mutation-positive advanced NSCLC6. Clinical trials have consistently demonstrated superior clinical activity and relative safety of osimertinib in advanced NSCLC patients with EGFR mutations regardless of their EGFR T790M mutation status7,8.
Most common adverse reactions of osimertinib (≥ 20%) were diarrhea, rash, dry skin, nail toxicity, and fatigue. Furthermore, the most frequent adverse reactions leading to dose reductions or interruptions were prolongation of the QT interval as assessed by electrocardiogram (ECG), neutropenia, and diarrhea6. Based on animal studies, osimertinib caused post-implantation loss, early embryonic death and caused an increase in total litter loss and postnatal death, which may impair fertility in females and males of reproductive potential7,8. Because clinical trials are conducted under widely varying conditions, adverse reactions observed in clinical trials may not reflect the real-world situation observed in practice. However, a meta-analysis showed that osimertinib notably increased the risk of cardiac toxicities9. Therefore, using data mining algorithm to search for the potential ADRs signals of osimertinib in the real-world is necessary for the study of osimertinib.
FDA Adverse Event Reporting System (FAERS) is a public database which is designed to facilitate the FDA’s post-marketing safety monitoring of drug and therapeutic products, and it is one of the largest pharmacovigilance databases in the world10,11. A FAERS study revealed the incidence of cardiotoxicity due to osimertinib compared with other drugs approved by the FDA and also specifically versus other EGFR-TKIs between January 1, 2016, and September 30, 2018, and mainly focused on the cardiotoxicity of osimertinib12. Data are lacking regarding the real-world safety of osimertinib from 2019 to 2021. In the present study, we retrospectively excavated and analyzed the AEs of osimertinib by data mining in FAERS from the first quarter of 2016 to the fourth quarter of 2021. Our study results may offer a guide for physicians and health policymakers to monitor ADRs for facilitating the rational use of clinical drugs.
Results
General characteristics
The clinical characteristics of osimertinib-associated AEs were described in Table 1. For gender, the incidence of AEs in females (55.45%) accounted for a larger proportion than males. In terms of age composition, patients whose age were over 65 years accounted for a higher proportion (36.98%) than patients under 18 years old and patients whose age between 18 and 65 years old. Non-small cell lung cancer was the most reported indication (49.95%), followed by lung neoplasm malignant (36.98%). US (48.20%) reported the largest number of AEs, followed by Japan (15.93%), China (5.70%), France (3.10%), and Thailand (2.42%). Serious outcomes include death, life-threatening, hospitalization, disability, and other serious outcomes. We divide one of them by all serious outcomes reports, to get the proportion. Death (45.85%) was the most frequently reported serious outcome which might be related to disease progression caused by tumor. Other serious outcomes and hospitalization were reported in 3425 (31.81%) and 1973 (18.33%) cases, respectively. Excluding the unknown reporters, physicians and consumers reported the most AEs in 28.11% and 24.73%, respectively. The number of AEs reported were increasing year by year, and the most reported year was 2021 (31.06%), followed by 2020 (27.90%), 2019 (17.60%), 2018 (10.51%), 2017 (8.10%), and 2016 (4.83%), respectively.
Signal detection
Signal strengths reports of osimertinib at the System Organ Class (SOC) level are described in Table 2. According to the statistics, we found that 27 organ systems were involved in osimertinib-induced AEs. The significant SOCs that met four criteria were neoplasms benign, malignant and unspecified (incl cysts and polyps) and congenital, familial and genetic disorders. Also, general disorders and administration site conditions, respiratory, thoracic and mediastinal disorders, and hepatobiliary disorders were significant SOCs that at least one of the four indices met the criteria.
After excluding neoplasms benign, malignant and unspecified (incl cysts and polyps) which may cause by the disease progression, totally 68 significant disproportionality PTs conforming to the four algorithms simultaneously are shown in Table 3. According to the previous study of osimertinib, cardiotoxicity events, pneumonitis, eye disorders, and skin disease events are usually reported. In our study, long QT syndrome (PT: 10024803), cardiac failure (PT: 10007554), cardiomyopathy (PT: 10007636), platelet count decreased (PT: 10035528), paronychia (PT: 10034016), etc. are consistent with findings from clinical trials. Interestingly, unexpected significant AEs were uncovered in the label, including B-Raf proto-oncogene, serine/threonine kinase (BRAF) gene mutation (PT:10075648), volvulus (PT: 10047697), mechanical ileus (PT: 10051399), amylase increased (PT: 10002016), immobilisation syndrome (PT: 10084349), cerebral infarction (PT: 10008118), deep vein thrombosis (PT: 10051055), venous thrombosis limb (PT: 10061408)
Onset time of events
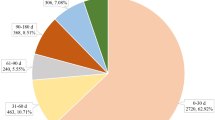
The onset times of osimertinib-associated AEs were collected from the database. Excluding false reports, totally 3841 AEs reported onset time. The median onset time was 58 days (interquartile range [IQR] 14–212 days). As shown in Fig. 1, results indicated that the onsets of osimertinib were general, cases may cover over a year. However, most of the cases occurred within the first month (n = 1460, 38.01%) after osimertinib initiation. However, the case ratios occurred in the 2 months (n = 488, 12.71%), 6 months (n = 498, 12.97%), and 12 months (n = 476, 12.39%) were similar, which reflected that AEs might occur at anytime within a year. Furthermore, AEs occurred after 1 year of osimertinib treatment with percentage of 15.78% (n = 606) as illustrated in our data.
Conclusion
Our pharmacovigilance analysis of FAERS database revealed the safety signals of osimertinib and time to AEs onsets with osimertinib comprehensively and systematically. New and unexpected significant AEs as volvulus, hepatic function abnormal, and VTEs might also occur. High attention should be paid to common AEs included long QT syndrome, endocrine, pneumonitis, and cardiomyopathy. Completely monitoring and risk identification of all these AEs are suggested in all populations. Cohort studies and long-term clinical investigations are still needed to verified these results and to further comprehend the safety of osimertinib.
Discussion
In previous studies, the research on osimertinib mostly focused on the mechanism, clinical trials, and literature analysis, etc., and few articles concentrated on the latest real-world research. Based on the largest samples of the real-world data, we collected and evaluated the pharmacovigilance of osimertinib in the post-market. The purpose is to analyze new and meaningful adverse reactions, to guide the update of summary of product characteristics (SmPC), and to provide a basis for clinical rational drug use.
The AEs of osimertinib occurred more commonly in females (55.45%) than in males (29.94%). This may be related to the increase of female patients with lung cancer, which leads to the increase of drug use opportunities. Studies have shown that females are more likely than males to have non-smoking lung cancer13. The causes of lung cancer in female may be related to the influence of estrogen14, environment15 and molecular factors16. Also, the study described a higher AEs proportion in elderly patients (36.98% patients > 65 years), which was consistent with the FLAURA and AURA clinical trials that the number of AEs leading to the dose adjustment (suspension or reduction) of the study drug was higher in subjects aged over 65 years6. With the increasing clinical application of osimertinib, it is important for clinicians to be alert to the AEs associated with osimertinib, especially in olderly patients. Early recognition of AEs is necessary because these effects can be life-threatening or lead to disease progression.
According to the disproportionality analysis, the most commonly reported and significant signals at SOC levels were general disorders and administration site conditions, and neoplasms benign, malignant and unspecified (incl cysts and polyps). These AEs included death, disease progression, malignant neoplasm progression, et al., which were not recorded in the SmPC of osimertinib and might be related to the patient's own disease progression rather than the drug itself. Evidence suggested that approximately 38% of all dead lung cancer patients had single site metastasis and 19% had two or more metastases17. It might be irrational to judge whether tumor metastasis and tumor progression were caused by osimertinib only by ADR signals. Clinicians should distinguish whether tumor-related disease is caused by osimertinib. Besides, significant signals were observed in SOC of respiratory, thoracic, and mediastinal disorders. Relevant studies have pointed out that different EGFR-TKIs can cause respiratory toxicity, and the incidence of interstitial lung disease (ILD) is 0–5.3%, while there is no significant difference between osimertinib and the first two generations18,19. Blood and lymphatic system disorders which related to the laboratory abnormalities were commonly found in more than 20% of patients in clinical trial6. However, AEs included hepatobiliary disorders, congenital, familial and genetic disorders are not mentioned in the SmPC. We should pay attention to whether they have clinical significance to guide clinical medication.
Among all the AEs, the AEs involving respiratory system and cardiovascular system still deserve attention. The respiratory adverse reactions mentioned in the SmPC of osimertinib include cough, pneumonia, and ILD. This study also excavated signs of AEs such as pneumothorax, pleural effusion, and hydrothorax. And the most common AEs leading to discontinuation of osimertinib was ILD/pneumonitis. Fan et al.18 found that all EGFR-TKIs had drug-related toxicities included ILD, and the incidence of drug-related ILD in different EGFR-TKIs ranged from 0 to 5.3%. The mechanism of ILD may be different for third-generation EGFR-TKIs, because osimertinib induced ILD in patients who had no pulmonary toxicities during a prior treatment with first- or second-generation EGFR-TKI19. The mechanism of osimertinib induced respiratory toxicity is not clear, but it may be related to the inhibition of the maintenance of epithelial cells. Osimertinib changed the expression of cytokines by impairing the growth and migration of epithelial cells, resulting in inflammatory cell recruitment and lung tissue injury20. Although different EGFR-TKIs could cause respiratory toxicity, the AEs related to EGFR-TKI were usually tolerable and controllable. Risk factors, such as tobacco exposure, pre-existing lung fibrosis, and chronic obstructive pulmonary disease, indicate that lung inflammatory circumstances may worsen with EGFR-TKI treatment because of impaired epithelial healing of lung injuries20. Noonan et al.21 demonstrated NSCLC patients who had previously received chest radiotherapy or had a history of aspiration were more likely to have lung shadows or subpleural nodules after using osimertinib. Furthermore, a combination of drugs with or without radiotherapy can increase the risk of ILD22. Therefore, we speculated that patients with chronic lung injury in the past were the high-risk population of respiratory toxicity. Mamesaya et al.23 reported a case of a 38-year-old female patient with osimertinib-induced ILD after treatment with anti-PD1 antibody and speculated anti-PD1 therapies might be the risk factor of EGFR-TKI-induced ILD. Our study suggests that for patients with chronic respiratory diseases and combined PD-1/PD-L1 therapy, the pharmaceutical care of osimertinib should be strengthened.
Cardiotoxicity is accompanied by a history of treatment with antitumor drugs. Whether traditional chemotherapy24, new targeted therapy25 or immunotherapy26 can cause cardiac related AEs. Cardiac related AEs are a commom toxicity of TKIs existed in not only first and second generation but third generation12,27. Our study not only found out AEs signals of cardiac disorders like heart failure, QT interval prolongation, electrocardiogram QT prolonged, in the SmPC of osimertinib, but also excavated AEs like ventricular dysfunction, cardiology, cardiac dysfunction and cardiotoxicity that do not exist in the SmPC. In vitro, osimertinib not only inhibited EGFR but also human epidermal growth factor receptor-2 (HER2) at clinically relevant concentrations. The current mainstream view is that the inhibition of HER2 is the main reason for the cardiotoxicity of some antitumor drugs28. HER2 is essential for maintaining cardiac function, and HER2 inhibition is the main cause of cardiotoxicity for some antitumor drugs. Trastuzumab is a monoclonal antibody targeting HER2, whose cardiotoxicity increased by 2.45 times after treatment29. Perez et al.30 also found that osimertinib might lead to dose independent reversible myocardial injury by inhibiting erythroblastic leukemia viral oncogene B (commonly referred to as HER). Kunimasa et al.31 found severe osimertinib-associated cardiotoxicities with a higher frequency (4.1%) than previous studies. They also suggested that in the clinical application of osimertinib, attention should be paid not only to the QTc interval prolongation, but also to other cardiotoxicity. Although osimertinib was highly specific for EGFR, study showed that osimertinib has a greater inhibitory effect on HER2 than other EGFR-TKIs32. Furthermore, rates of QT prolongation, cardiac failure, and atrial fibrillation were found to be higher when osimertinib compared with other EGFR-TKIs in FAERS12. Based on all the facts we speculated that HER2 was the main cause of osimertinib cardiotoxicity. The risk factors of cardiotoxicity caused by antitumor drugs include age, potential heart disease, renal insufficiency, and the combination of other cardiotoxic drugs, while the risk of cardiotoxicity caused by EGFR-TKI is more closely related to the patient's cardiovascular history33. Thus, the early awareness of cardiotoxicities, monitoring for QT prolongation, managing symptoms of heart failure, and close follow-up, may enhance the benefits of therapy while taking osimertinib.
Excitingly, we found some unexpected and significant safety signals, which included BRAF V600E mutation positive, volvulus, hepatic function abnormal, and venous thromboembolism (VTE). BRAF is an important proto oncogene in human beings. About 15% of malignant tumors are related to BRAF mutation34. At present, there are many mutations in this gene, of which BRAF V600E is the most common accounts for 75–82% of BRAF mutations in cutaneous melanoma35. Different from EGFR and ALK gene mutations, BRAF V600 mutation is relatively rare in non-small cell lung cancer, about 2–3% of which are adenocarcinoma36,37. BRAF mutation will continuously activate the downstream MEK-ERK signal pathway and play a vital role in tumor growth, proliferation, invasion, and metastasis. There is no specific treatment for NSCLC patients carrying a BRAF mutation, even if in melanoma, BRAF inhibitors were demonstrated to prolong progression-free survival and survival38. Therefore, BRAF mutation represents strong tumor invasiveness. Therefore, we infer that BRAF mutation may be a signal reflecting disease progression.
Drug-induced liver injury is an important adverse effect of TKIs. In vitro, osimertinib was mainly eliminated by the liver and metabolized by Cytochrome P450 (CYP, P450) 3A4 and CYP 3A5. The main metabolic pathways are oxidation and dealkylation. In the AURA2 study, 1 patient developed drug induced liver injury (DILI) which was manifested by elevated serum aminotransferase levels (< 1%)39. Elevated liver transaminases (all grades) associated with EGFR-TKI use are seen in 25–55%, 27–38%, 10%, and 9% of patients treated with gefitinib, erlotinib, afatinib, and osimertinib, respectively. Severely (grade 3 or 4) elevated liver transaminases is found in 1% of patients treated with osimertinib7. The mechanism of liver injury caused by EGFR-TKI has not been fully elucidated. Some researchers believe that the liver toxicity of TKI drugs is related to the metabolism of their active metabolites, which can interfere with cellular molecules and thus affect cell function and death40. Autoimmune activation is also a mechanism by which TKIs cause hepatotoxicity41. Ivan Gonzalez et al.,42 reported that pericentral confluent necrosis and parenchymal collapse in liver biopsy after the patient treated with osimertinib and developed transaminitis of unclear etiology, which has been reported in other TKIs. Hirabayashi et al.43, reported a case that osimertinib induced hepatotoxicity after 15 days of treatment. Although there are not many case reports about liver injury, it is undeniable that it has become a class of AEs that needs to be paid enough attention to liver disorders are often improved with dose reduction or transient discontinuation of EGFR-TKIs, and concomitant use of hepatoprotective agents17.
Nowadays, there has been no association with volvulus and EGFR inhibition. The occurrence of volvulus is caused by a variety of reasons, and physiological or pathological factors are the predisposing factors. The risk factors of cecal volvulus included chronic constipation, distal colon obstruction, high-fiber diets, ileus, prior colonoscopy, and late pregnancy44. Constipation, a known risk factor for volvulus, was only found in 7 cases in clinical trial. Patil et al.45, reported 3 cases associating cecal volvulus with the 160 mg dose of osimertinib, and highlighted a potentially vital surgical complication associated with the 160 mg dose of osimertinib.
Tumor patients have a higher incidence of VTE than normal people. The mortality of tumor patients with VTE is a twofold increased mortality rate compared to cancer patients without VTE46. Accurate assessment of patients' risk of VTE can effectively prevent the occurrence of VTE events and reduce mortality. According to our study, osimertinib can also lead to deep vein thrombosis, venous thrombosis limb, and pulmonary embolism. A meta-analysis of venous thromboembolic events associated with VEGFR-TKIs found that the use of VEGFR-TKIs does not significantly increase the risk of VTEs, the risk of VTEs in cancer patients is mainly affected by tumor types, host factors, and concomitant usage of anticancer drugs47. Hotta et al.48, aimed to identify anticancer drugs and anticoagulants that can be used safely in combination, as accompanying study to an observational research on VTE incidence rates in lung cancer patients. And the study indicated that the PK of anticoagulants was not affected by co-administration of EGFR-TKIs (gefitinib, erlotinib, and afatinib). While, early diagnosis, appropriate treatment, and prevention are considered important measures to improve prognosis.
Results of our study showed that the median onset time was 58 days, and most cases occurred within the first month (n = 1460, 38.01%), after osimertinib initiation. In FLAURA and AURA, 49% of patients reported diarrhea with a median duration of 19 and 22 days and a median duration of 19 and 6 days, respectively. We also found that except for the first month, the probability of AEs within one year was similar. The median time of ILD or ILD like adverse reactions in the global population is 85 days. These results suggested that we should pay special attention to the AEs of patients in the first month and early recognition of AEs caused by osimertinib therapy could reduce the agony of patients which can be life-threatening.
Based on FAERS database, our study excavates and analyzes the adverse reaction signals of osimertinib, discusses the respiratory toxicity and cardiotoxicity related to osimertinib, and some other meaningful AEs, in order to provide some reference for improving the safety of clinical medication. FAERS database is a spontaneous reporting system. Due to its own limitations, there are phenomena such as underreporting, re-reporting, incomplete case information and so on. And lack of underlying disease and concomitant medication may affect the results. Moreover, media attention and recent publication of an adverse drug reaction in the literature might affect the reporting behaviors49. However, despite the facts that FAERS database has some limitations in pharmacovigilance studies, a comprehensive characterization of the AE signals from osimertinib and the discovery of some unexpected AE signals might provide foundation for further clinical studies of osimertinib. And the efficacy and safety of osimertinib still need to be continuously monitored.
Methods
Data source and collection
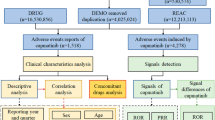
We launched a pharmacovigilance study of osimertinib in the post-marketing setting using data covering the period from first quarter of 2016 to the fourth quarter of 2021 from the FAERS database. FAERS database is based on the International Safety Reporting Guidelines (ICH E2B) issued by ICH, and the adverse events are coded according to the Medical Dictionary for Regulatory Activities (MedDRA). Seven databases make up FAERS data files, including demographic and administrative information (DEMO), adverse drug reaction information (REAC), patient outcome information (OUTC), drug information (DRUG), drug therapy starts dates and end dates (THER), information on report sources (RPSR), and indications for use/diagnosis (INDI). We chose the latest FDA_DT with the same CASEID or selected the higher PRIMARYID when the CASEID and FDA_DT were the same to identify and remove duplicate reports. During the study period, totally 9,704,338 reports of osimertinib were gained from FAERS database. 8,379,682 case reports of osimertinib as the primary suspect (PS) drug after the exclusion of duplicates, and 10,804 AEs were associated with osimertinib (Fig. 2). All AEs reports of osimertinib were identified in system organ class (SOC) and PT levels. The codes of drugs reported in event include PS, secondary suspect drug (SS), concomitant (C), and interacting (I). Moreover, generic name (Osimertinib) and trade name (Tagrisso) were defined as target drugs in the DRUG file, and we chose the role_cod as PS.
Statistical analysis
The association between osimertinib and AEs were determined by the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN) and the multi-item gamma Poisson shrinker (MGPS) algorithms, which was based on the disproportionality analysis50. The equations and criteria for the four algorithms are described in Supplementary Table S1. The data that were chosen for analysis in our study were AE signals that met four algorithm standards. The novelty signals are identified as any significant AE which was not listed in package inserts51.
The onset time was defined as the interval between EVENT_DT (date of adverse event occurrence) and START_DT (start date for osimertinib use). Moreover, input errors (EVENT_DT earlier than START_DT) reports or inaccurate date entries were excluded. The time-to-onset was describled by median and interquartile ranges (IQR). MYSQL 8.0, Navicat Premium 15, Microsoft EXCEL 2016 and the GraphPad Prism 8 (GraphPad Software, CA, USA) were used to perform data processing and statistical analyses.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Stinchcombe, T. E. & SocinskI, M. A. Considerations for second-line therapy of non-small cell lung cancer. Oncologist 13, 28–36 (2008).
Mountain, C. F. Revisions in the international system for staging lung. Cancer 11, 1710–1717 (2010).
Guo, X. et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer 127, 84–89 (2019).
Wu, S. G. et al. An observational retrospective study to evaluate the incidence of acquired EGFR T790M resistance in NSCLC patients with EGFR mutation following progression after at least one prior EGFR TKI treatment in Taiwan: ARISE study-Science Direct. Ann. Oncol. 30, 168 (2019).
Hochmair, M. J. et al. Overall survival in patients with EGFRm+ NSCLC receiving sequential afatinib and osimertinib: Updated analysis of the GioTag study. Ann. Oncol. 30, 165 (2019).
Astrazeneca pharmaceuticals. Tagrisso (osimertinib) [package insert]. U.S. Food and Drug Administration website. Revised April 2018. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf.
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2020).
Thein, K. Z., Swarup, S., Ball, S., Quirch, M., Vorakunthada, Y., Htwe, K. K., et al. 1388p incidence of cardiac toxicities in patients with advanced non-small cell lung cancer treated with osimertinib: A combined analysis of two phase III randomized controlled trials. Ann. Oncol. 29, suppl_8 (2018).
Chen, C. et al. Immune-related adverse events associated with immune checkpoint inhibitors: An updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int. Immunopharmacol. 95, 107498 (2021).
Meng, L. et al. Assessing fluoroquinolone-associated aortic aneurysm and dissection: Data mining of the public version of the FDA adverse event reporting system. Int. J. Clin. Pract. 73, e13331 (2019).
Anand, K., Ensor, J., Trachtenberg, B., et al. Osimertinib induced cardio-toxicity: A retrospective review of FDA adverse events reporting system (FAERS). J. Clin. Oncol. 37(15_suppl), 9044 (2019).
Yuliang, S. et al. DNA repair gene polymorphisms in relation to non-small cell lung cancer survival. Cell Physiol. Biochem. 36, 1419–1429 (2015).
Pietras, R. J. et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 70, 372–381 (2005).
Lam, W. Lung cancer in Asian women-the environment and genes. Respirology 10, 408–417 (2010).
Yokota, J. & Kohno, T. Molecular footprints of human lung cancer progression. Cancer Sci. 95, 197–204 (2010).
Riihim, K. et al. Metastatic sites and survival in lung cancer. Lung Cancer 86, 78 (2014).
Fan, M., Mo, T., Shen, L. & Yang, L. Osimertinib-induced severe interstitial lung disease: A case report. Thoracic Cancer. 10, 1657–1660 (2019).
Matsumoto, Y. et al. Interstitial lung disease induced by osimertinib for Epidermal Growth Factor Receptor (EGFR) T790M-positive Non-small Cell Lung Cancer. Intern. Med. 56, 2325–2328 (2017).
Ohmori, T., Yamaoka, T., Ando, K., Kusumoto, S. & Sagara, H. Molecular and clinical features of egfr-tki-associated lung injury. Int. J. Mol. Sci. 22, 792 (2021).
Noonan, S. A., Sachs, P. B. & Camidge, D. R. Transient asymptomatic pulmonary opacities occurring during osimertinib treatment. J. Thorac. Oncol. 11, 2253–2258 (2016).
Camus, P., Kudoh, S. & Ebina, M. Interstitial lung disease associated with drug therapy. Br. J. Cancer. 91(Suppl 2), 18–23 (2004).
Mamesaya, N. et al. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Investig. New Drugs 35, 105–107 (2016).
Dessalvi, C. C. et al. Antioxidant approach as a cardioprotective Strategy in chemotherapy-Induced cardiotoxicity. Antioxid. Redox. Signal. 34, 572–588 (2020).
Tajiri, K., Aonuma, K. & Sekine, I. Cardiovascular toxic effects of targeted cancer therapy. Jpn. J. Clin. Oncol. 47, 779–785 (2017).
Knobloch, K. et al. Simultaneous hemodynamic and serological cardiotoxicity monitoring during immunotherapy with trastuzumab. Int. J. Cardiol. 125, 113–115 (2008).
Alhoshani, A. et al. EGFR inhibitor gefitinib induces cardiotoxicity through the modulation of cardiac PTEN/Akt/FoxO3a pathway and reactive metabolites formation: In vivo and in vitro rat studies. Chem. Res. Toxicol. 33, 1719–1728 (2020).
Pondé, N. F., Lambertini, M. & Azambuja, E. D. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 1, e000073 (2016).
Watanabe, H. et al. Congestive heart failure during osimertinib treatment for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC). Intern. Med. 56, 2195–2197 (2017).
Perez, I. E. et al. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin. Med. Insights Cardiol. 13, 117954681986644–117954681986644 (2019).
Kunimasa, K., Kimura, M., Oboshi, M., et al. MO2–16–2 Irreversible severe cardiotoxicities except for QTc interval prolongation associated with Osimertinib. Ann. Oncol. 30, vi109 (2019)
Piper-Vallillo, A. J. et al. Heart failure associated with the epidermal growth factor receptor inhibitor osime rtinib. JACC Cardio Oncol. 2, 119–122 (2020).
Sadasivan, C. et al. Cardiovascular toxicity of PI3Kα inhibitors. Clin. Sci. (Lond.) 134, 2595–2622 (2020).
Seghers, A. K., Cuyle, P. J. & Cutsem, E. V. Molecular targeting of a BRAF mutation in pancreatic ductal adenocarcinoma: Case report and literature review. Target Oncol. 15, 407–410 (2020).
Pisareva, E. et al. Sensitive allele-specific real-time PCR test for mutations in BRAF codon V600 in skin melanoma. Melanoma Res. 24, 322–331 (2014).
Brose, M. S. et al. BRAFand KRAS mutations in human lung cancer and melanoma. Cancer Res. 62, 6997–7000 (2002).
Naoki, K. et al. Missense mutations of BRAF gene in human lung adenocarcinoma. Cancer Res. 62, 7001–7003 (2002).
Arcangelo, M., Incecco, A. & Cappuzzo, F. Rare mutations in non-small-cell lung cancer. Future Oncol. 9, 699–711 (2013).
Goss, G. et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 17, 1643–1652 (2016).
Teng, W. C. et al. Mechanism-based inactivation of cytochrome P450 3A4 by lapatinib. Mol. Pharmacol. 78, 693–703 (2010).
Spraggs, C. F. et al. HLA-DQA1/02:01 is amajor risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 29, 667–673 (2011).
González, I. & Chatterjee, D. Histopathological features of drug-induced liver injury secondary to osimertinib. ACG Case Rep. J. 6, 1–3 (2019).
Hirabayashi, R., Fujimoto, D., Satsuma, Y., Hirabatake, M. & Tomii, K. Successful oral desensitization with osimertinib following osimertinib induced fever and hepatotoxicity: A case report. Investig. New Drugs 36, 952–954 (2018).
Gingold, D. & Murrell, Z. Management of colonic volvulus. Clin. Colon Rectal Surg. 25, 236–244 (2012).
Patil, T. et al. Cecal volvulus as a rare complication of osimertinib dosed at 160 mg in patients with EGFR-mutant non-small cell lung cancer. Front Oncol. 10, 510 (2020).
Lyman, G. H., Bohlke, K., Khorana, A. A., Kuderer, N. M., Lee, A. Y., et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical Oncology clinical practice guideline update 2014. J. Clin. Oncol. 33, 654e6 (2015).
Qi, W. X. et al. Risk of venous thromboembolic events associated with VEGFR-TKIs: A systematic review and meta-analysis. Int. J. Cancer 132, 2967–2974 (2012).
Hotta, T. et al. Pharmacokinetics of edoxaban in EGFR-mutated non-small cell lung cancer patients with venous thromboembolism. Respir. Investig. 59, 327–334 (2021).
Teng, C. & Frei, C. R. Delirium associations with antibiotics: a pharmacovigilance study of the FDA Adverse Event Reporting System (FAERS). Drugs Real World Outcomes 9, 23–29 (2022).
Shu, Y. et al. Post-marketing safety concerns with secukinumab: A disproportionality analysis of the FDA Adverse Event Reporting System. Front Pharmacol. 13, 862508 (2022).
Shu, Y., He, X., Liu, Y., Wu, P. & Zhang, Q. A real-world disproportionality analysis of olaparib: Data mining of the public version of FDA Adverse Event Reporting System. Clin. Epidemiol. 14, 789–802 (2022).
Author information
Authors and Affiliations
Contributions
F.L., and J.L. contributed to conception and study design, and took responsibility for the collection, integrity and accuracy of the data. Y.Y., drafted the manuscript, Y.S., J.Z., participated in data analyses and interpretation, and revisions of the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, Y., Shu, Y., Zhu, J. et al. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci Rep 12, 19555 (2022). https://doi.org/10.1038/s41598-022-23834-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23834-1
- Springer Nature Limited
This article is cited by
-
Synergy of de-walled Ganoderma Lucidum spore powder (GLSP) on targeted therapy in advanced non-squamous non-small cell lung cancer with epidermal growth factor receptor (EGFR) mutant: protocol for a randomized, double-blind, placebo-controlled study
BMC Complementary Medicine and Therapies (2024)
-
A real-world pharmacovigilance study of FDA adverse event reporting system events for Capmatinib
Scientific Reports (2024)
-
Thromboembolic Events Associated with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Pharmacovigilance Analysis of the US FDA Adverse Event Reporting System (FAERS) Database
Clinical Drug Investigation (2024)