Abstract
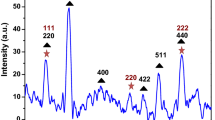
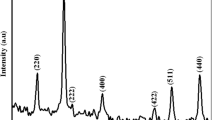
In the present study, the synthesis of metal organic framework (MOF) template-derived materials is reported. Cubic Fe2O3·TiO2 material was synthesized by using Prussian blue as the sacrificed template and aqueous soluble TiOSO4 as a TiO2 precursor. The obtained material was characterized by Fourier transform infrared (FT-IR) and Raman spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), ultraviolet visible diffuse reflectance spectroscopy (UV-Vis DRS), and nitrogen adsorption/desorption isotherms. The characterization confirmed the existence of cubic α-Fe2O3 core and anatase TiO2 shell in the composite. Furthermore, the appearance of TiO2 shell has significantly enhanced the Brunauer–Emmett–Teller (BET) surface area yet still retained small bandgap energy of around 2.0 eV. This material was employed to degrade chosen organic dyes, including cationic dyes and anionic dye, in a heterogeneous photo-Fenton like system. The experimental results showed that this material exhibited higher adsorption and degradation capacity toward cationic dyes than anionic dye. The fitting of experimental data into two kinetic models revealed that the removal of the dyes can be better described by Langmuir-Hinshelwood model. The recyclability of the catalyst was also examined.
Graphical Abstract














Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on a reasonable request.
References
Kim JY et al (2017) Exploiting diffusion barrier and chemical affinity of metal–organic frameworks for efficient hydrogen isotope separation. J Am Chem Soc 139(42):15135–15141. https://doi.org/10.1021/jacs.7b07925
Li J-R et al (2011) Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord Chem Rev 255(15):1791–1823. https://doi.org/10.1016/j.ccr.2011.02.012
Murray LJ, Dincă M, Long JR (2009) Hydrogen storage in metal–organic frameworks. Chem Soc Rev 38(5):1294–1314. https://doi.org/10.1039/B802256A
Lin R-B, Li F, Liu S-Y, Qi X-L, Zhang J-P, Chen X-M (2013) A noble-metal-free porous coordination framework with exceptional sensing efficiency for oxygen. Angew Chem Int Ed 52(50):13429–13433. https://doi.org/10.1002/anie.201307217
Lustig WP, Mukherjee S, Rudd ND, Desai AV, Li J, Ghosh SK (2017) Metal–organic frameworks: functional luminescent and photonic materials for sensing applications. Chem Soc Rev 46(11):3242–3285. https://doi.org/10.1039/C6CS00930A
Yi F-Y, Chen D, Wu M-K, Han L, Jiang H-L (2016) Chemical sensors based on metal–organic frameworks. ChemPlusChem 81(8):675–690. https://doi.org/10.1002/cplu.201600137
Sun J-K, Xu Q (2014) Functional materials derived from open framework templates/precursors: synthesis and applications. Energy Environ Sci 7(7):2071–2100. https://doi.org/10.1039/C4EE00517A
Wang H, Zhu Q-L, Zou R, Xu Q (2017) Metal-organic frameworks for energy applications. Chem 2(1):52–80. https://doi.org/10.1016/j.chempr.2016.12.002
Zhao S-N, Song X-Z, Song S-Y, Zhang H-J (2017) Highly efficient heterogeneous catalytic materials derived from metal-organic framework supports/precursors. Coord Chem Rev 337:80–96. https://doi.org/10.1016/j.ccr.2017.02.010
Xia Q, Li Z, Tan C, Liu Y, Gong W, Cui Y (2017) Multivariate metal–organic frameworks as multifunctional heterogeneous asymmetric catalysts for sequential reactions. J Am Chem Soc 139(24):8259–8266. https://doi.org/10.1021/jacs.7b03113
Huang G, Yang Q, Xu Q, Yu S-H, Jiang H-L (2016) Polydimethylsiloxane coating for a palladium/MOF composite: highly improved catalytic performance by surface hydrophobization. Angew Chem Int Ed 55(26):7379–7383. https://doi.org/10.1002/anie.201600497
Li B, Chrzanowski M, Zhang Y, Ma S (2016) Applications of metal-organic frameworks featuring multi-functional sites. Coord Chem Rev 307:106–129. https://doi.org/10.1016/j.ccr.2015.05.005
Yang Q, Xu Q, Yu S-H, Jiang H-L (2016) Pd nanocubes@ZIF-8: integration of plasmon-driven photothermal conversion with a metal–organic framework for efficient and selective catalysis. Angew Chem Int Ed 55(11):3685–3689. https://doi.org/10.1002/anie.201510655
Chen Y-Z, Zhang R, Jiao L, Jiang H-L (2018) Metal–organic framework-derived porous materials for catalysis. Coord Chem Rev 362:1–23. https://doi.org/10.1016/j.ccr.2018.02.008
Wu Q, Wu G, Wang L, Hu W, Wu H (2015) Facile synthesis and optical properties of Prussian Blue microcubes and hollow Fe2O3 microboxes. Mater Sci Semicond Process 30:476–481. https://doi.org/10.1016/j.mssp.2014.10.014
Xue Z, Li L, Cao L, Zheng W, Yang W, Yu X (2020) A simple method to fabricate NiFe2O4/NiO@Fe2O3 core-shelled nanocubes based on Prussian blue analogues for lithium ion battery. J Alloys Compd 825:153966. https://doi.org/10.1016/j.jallcom.2020.153966
Li X et al (2018) Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem Eng J 336:241–252. https://doi.org/10.1016/j.cej.2017.11.188
Zhao C, Shen C, Xin F, Sun Z, Han W (2014) Prussian blue-derived Fe2O3/sulfur composite cathode for lithium–sulfur batteries. Mater Lett 137:52–55. https://doi.org/10.1016/j.matlet.2014.08.115
Cheng Z et al (2020) Metal organic framework-derived porous Fe2N nanocubes by rapid-nitridation for efficient photocatalytic hydrogen evolution. Mater Adv 1(5):1161–1167. https://doi.org/10.1039/D0MA00074D
Guo Y, Tian X, Wang X, Sun J (2019) Fe2O3 nanomaterials derived from Prussian blue with excellent H2S sensing properties. Sensors Actuators B Chem 293:136–143. https://doi.org/10.1016/j.snb.2019.04.027
Teng Q et al (2018) Formation of Fe2O3 microboxes/ macroporous carbon hybrids from Prussian blue template for electrochemical applications. J Alloys Compd 739:425–430. https://doi.org/10.1016/j.jallcom.2017.12.291
Zhang L, Wu HB, Madhavi S, Hng HH, Lou XW (2012) Formation of Fe2O3 microboxes with hierarchical shell structures from metal–organic frameworks and their lithium storage properties. J Am Chem Soc 134(42):17388–17391. https://doi.org/10.1021/ja307475c
Wang N, Du Y, Ma W, Xu P, Han X (2017) Rational design and synthesis of SnO2-encapsulated α-Fe2O3 nanocubes as a robust and stable photo-Fenton catalyst. Appl Catal B Environ 210:23–33. https://doi.org/10.1016/j.apcatb.2017.03.037
Cheng L, Qiu S, Chen J, Shao J, Cao S (2017) A practical pathway for the preparation of Fe2O3 decorated TiO2 photocatalyst with enhanced visible-light photoactivity. Mater Chem Phys 190:53–61. https://doi.org/10.1016/j.matchemphys.2017.01.001
Zheng X, Fu W, Kang F, Peng H, Wen J (2018) Enhanced photo-Fenton degradation of tetracycline using TiO2-coated α-Fe2O3 core–shell heterojunction. J Ind Eng Chem 68:14–23. https://doi.org/10.1016/j.jiec.2018.07.024
Liu J et al (2015) 3D Flowerlike α-Fe2O3@TiO2 core–shell nanostructures: general synthesis and enhanced photocatalytic performance. ACS Sustain Chem Eng 3(11):2975–2984. https://doi.org/10.1021/acssuschemeng.5b00956
Khasawneh OFS, Palaniandy P (2021) Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: a review. Environ Technol Innov 21:101230. https://doi.org/10.1016/j.eti.2020.101230
Yao K, Basnet P, Sessions H, Larsen GK, Murph SEH, Zhao Y (2016) Fe2O3–TiO2 core–shell nanorod arrays for visible light photocatalytic applications. Catal Today 270:51–58. https://doi.org/10.1016/j.cattod.2015.10.026
Xia Y, Yin L (2013) Core–shell structured α-Fe2O3@TiO2 nanocomposites with improved photocatalytic activity in the visible light region. Phys Chem Chem Phys 15(42):18627–18634. https://doi.org/10.1039/C3CP53178C
Niu Y, Li M, Jia X, Shi Z, Liu H, Zhang X (2022) Structures and photocatalytic activity of α-Fe2O3@TiO2 core - shell nanoparticles. Solid State Commun 345:114683. https://doi.org/10.1016/j.ssc.2022.114683
Khasawneh OFS, Palaniandy P, Ahmadipour M, Mohammadi H, Bin Hamdan MR (2021) Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J Environ Chem Eng 9(1):104921. https://doi.org/10.1016/j.jece.2020.104921
Chen X-L, Li F, Chen H, Wang H, Li G (2020) Fe2O3/TiO2 functionalized biochar as a heterogeneous catalyst for dyes degradation in water under Fenton processes. J Environ Chem Eng 8(4):103905. https://doi.org/10.1016/j.jece.2020.103905
Hassan ME, Chen Y, Liu G, Zhu D, Cai J (2016) Heterogeneous photo-Fenton degradation of methyl orange by Fe2O3/TiO2 nanoparticles under visible light. J Water Proc engineering 12:52–57. https://doi.org/10.1016/j.jwpe.2016.05.014
Zheng R, Yang D, Chen Y, Bian Z, Li H (2022) Fe2O3/TiO2/reduced graphene oxide-driven recycled visible-photocatalytic Fenton reactions to mineralize organic pollutants in a wide pH range. J Environ Sci. https://doi.org/10.1016/j.jes.2022.01.042
Tu TH et al (2020) Synthesis of Fe2O3/TiO2/graphene aerogel composite as an efficient Fenton-photocatalyst for removal of methylene blue from aqueous solution. Vietnam J Chem 58(5):697–704. https://doi.org/10.1002/vjch.202000109
Singh J, Sharma S, Aanchal, and S. Basu (2019) Synthesis of Fe2O3/TiO2 monoliths for the enhanced degradation of industrial dye and pesticide via photo-Fenton catalysis. J Photochem Photobiol A Chem 376:32–42. https://doi.org/10.1016/j.jphotochem.2019.03.004
Hernández-Coronado EE et al (2021) Effective degradation of cefuroxime by heterogeneous photo-Fenton under simulated solar radiation using α-Fe2O3-TiO2. J Environ Chem Eng 9(6):106822. https://doi.org/10.1016/j.jece.2021.106822
Behnajady MA, Eskandarloo H, Modirshahla N, Shokri M (2011) Sol-gel low-temperature synthesis of stable anatase-type TiO2 nanoparticles under different conditions and its photocatalytic activity. Photochem Photobiol 87(5):1002–1008. https://doi.org/10.1111/j.1751-1097.2011.00954.x
Wetchakun N, Phanichphant S (2008) Effect of temperature on the degree of anatase–rutile transformation in titanium dioxide nanoparticles synthesized by the modified sol–gel method. Curr Appl Phys 8(3):343–346. https://doi.org/10.1016/j.cap.2007.10.028
Ngamta S, Boonprakob N, Wetchakun N, Ounnunkad K, Phanichphant S, Inceesungvorn B (2013) A facile synthesis of nanocrystalline anatase TiO2 from TiOSO4 aqueous solution. Mater Lett 105:76–79. https://doi.org/10.1016/j.matlet.2013.04.064
Chowdhury IH, Ghosh S, Naskar MK (2016) Aqueous-based synthesis of mesoporous TiO2 and Ag–TiO2 nanopowders for efficient photodegradation of methylene blue. Ceram Int 42(2, Part A):2488–2496. https://doi.org/10.1016/j.ceramint.2015.10.049
Horti NC, Kamatagi MD, Patil NR, Nataraj SK, Sannaikar MS, Inamdar SR (2019) Synthesis and photoluminescence properties of titanium oxide (TiO2) nanoparticles: effect of calcination temperature. Optik 194:163070. https://doi.org/10.1016/j.ijleo.2019.163070
Ramalingam V, Sundaramahalingam S, Rajaram R (2019) Size-dependent antimycobacterial activity of titanium oxide nanoparticles against Mycobacterium tuberculosis. J Mater Chem B 7(27):4338–4346. https://doi.org/10.1039/C9TB00784A
Tyrpekl V, Vejpravová JP, Roca AG, Murafa N, Szatmary L, Nižňanský D (2011) Magnetically separable photocatalytic composite γ-Fe2O3@TiO2 synthesized by heterogeneous precipitation. Appl Surf Sci 257(11):4844–4848. https://doi.org/10.1016/j.apsusc.2010.12.110
Wang Q, Wang N, He S, Zhao J, Fang J, Shen W (2015) Simple synthesis of Prussian blue analogues in room temperature ionic liquid solution and their catalytic application in epoxidation of styrene. Dalton Trans 44(28):12878–12883. https://doi.org/10.1039/C5DT01762A
Lassoued A, Dkhil B, Gadri A, Ammar S (2017) Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys 7:3007–3015. https://doi.org/10.1016/j.rinp.2017.07.066
Zhu K-R, Zhang M-S, Chen Q, Yin Z (2005) Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys Lett A 340(1):220–227. https://doi.org/10.1016/j.physleta.2005.04.008
Choi HC, Jung YM, Kim SB (2005) Size effects in the Raman spectra of TiO2 nanoparticles. Vib Spectrosc 37(1):33–38. https://doi.org/10.1016/j.vibspec.2004.05.006
Mansour H et al (2017) Structural, optical, magnetic and electrical properties of hematite (α-Fe2O3) nanoparticles synthesized by two methods: polyol and precipitation. Appl Physics A 123(12):787. https://doi.org/10.1007/s00339-017-1408-1
Lu J-F, Tsai C-J (2014) Hydrothermal phase transformation of hematite to magnetite. Nanoscale Res Lett 9(1):230. https://doi.org/10.1186/1556-276X-9-230
Abdel-Wahab A-M, Al-Shirbini A-S, Mohamed O, Nasr O (2017) Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures. J Photochem Photobiol A Chem 347:186–198. https://doi.org/10.1016/j.jphotochem.2017.07.030
Idriss H (2021) On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf Sci 712:121894. https://doi.org/10.1016/j.susc.2021.121894
Chen C-C, Hu S-H, Fu Y-P (2015) Effects of surface hydroxyl group density on the photocatalytic activity of Fe3+-doped TiO2. J Alloys Compd 632:326–334. https://doi.org/10.1016/j.jallcom.2015.01.206
Zhong Y et al (2014) The constraints of transition metal substitutions (Ti, Cr, Mn, Co and Ni) in magnetite on its catalytic activity in heterogeneous Fenton and UV/Fenton reaction: From the perspective of hydroxyl radical generation. Appl Catal B Environ 150-151:612–618. https://doi.org/10.1016/j.apcatb.2014.01.007
Li L, Hu C, Zhang L, Shi B (2021) More octahedral Cu+ and surface acid sites in uniformly porous Cu-Al2O3 for enhanced Fenton catalytic performances. J Hazard Mater 406:124739. https://doi.org/10.1016/j.jhazmat.2020.124739
Chen M, Shen X, Wu Q, Li W, Diao G (2015) Template-assisted synthesis of core–shell α-Fe2O3@TiO2 nanorods and their photocatalytic property. J Mater Sci 50(11):4083–4094. https://doi.org/10.1007/s10853-015-8964-6
Yadav A et al (2019) Effect of graphene oxide loading on TiO2: Morphological, optical, interfacial charge dynamics-a combined experimental and theoretical study. Carbon 143:51–62. https://doi.org/10.1016/j.carbon.2018.10.090
Rabiee H, Farahani MHDA, Vatanpour V (2014) Preparation and characterization of emulsion poly(vinyl chloride) (EPVC)/TiO2 nanocomposite ultrafiltration membrane. J Membr Sci 472:185–193. https://doi.org/10.1016/j.memsci.2014.08.051
Li J-F, Xu Z-L, Yang H, Yu L-Y, Liu M (2009) Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl Surf Sci 255(9):4725–4732. https://doi.org/10.1016/j.apsusc.2008.07.139
Xia H, Wang Q (2002) Ultrasonic irradiation: a novel approach to prepare conductive polyaniline/nanocrystalline titanium oxide composites. Chem Mater 14(5):2158–2165. https://doi.org/10.1021/cm0109591
Yoshida M, Lal M, Kumar ND, Prasad PN (1997) TiO2 nano-particle-dispersed polyimide composite optical waveguide materials through reverse micelles. J Mater Sci 32(15):4047–4051. https://doi.org/10.1023/A:1018645722633
Yang S-T, Chen S, Chang Y, Cao A, Liu Y, Wang H (2011) Removal of methylene blue from aqueous solution by graphene oxide. J Colloid Interface Sci 359(1):24–29. https://doi.org/10.1016/j.jcis.2011.02.064
Cho HH, Wepasnick K, Smith BA, Bangash FK, Fairbrother DH, Ball WP (2010) Sorption of aqueous Zn[II] and Cd[II] by multiwall carbon nanotubes: the relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir 26(2):967–981. https://doi.org/10.1021/la902440u
Peng L, Xie T, Lu Y, Fan H, Wang D (2010) Synthesis, photoelectric properties and photocatalytic activity of the Fe2O3/TiO2 heterogeneous photocatalysts. Phys Chem Chem Phys 12(28):8033–8041. https://doi.org/10.1039/C002460K
Barbosa IA et al (2017) Magnetic diatomite(Kieselguhr)/Fe2O3/TiO2 composite as an efficient photo-Fenton system for dye degradation. Solid State Sci 72:14–20. https://doi.org/10.1016/j.solidstatesciences.2017.08.007
Kumar MRA, Abebe B, Nagaswarupa HP, Murthy HCA, Ravikumar CR, Sabir FK (2020) Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: its applications in dye decolorization and as supercapacitors. Sci Rep 10(1):1249. https://doi.org/10.1038/s41598-020-58110-7
Lin Z, Liu P, Yan J, Yang G (2015) Matching energy levels between TiO2 and α-Fe2O3 in a core–shell nanoparticle for visible-light photocatalysis. J Mater Chem A 3(28):14853–14863. https://doi.org/10.1039/C5TA02958A
Vorontsov AV (2019) Advancing Fenton and photo-Fenton water treatment through the catalyst design. J Hazard Mater 372:103–112. https://doi.org/10.1016/j.jhazmat.2018.04.033
Van Hung N et al (2021) Visible light photocatalytic degradation of organic dyes using W-modified TiO2/SiO2 catalyst. Vietnam J Chem 59(5):620–638. https://doi.org/10.1002/vjch.202100016
Funding
This study is funded by the Ministry of Education and Training, Vietnam (No. B2021-DNA-08).
Author information
Authors and Affiliations
Contributions
VTN: conceptualization, funding acquisition, writing—review, and editing. VTD: methodology, formal analysis, and writing—original draft. NTMB: investigation and data curation. TDM: conceptualization and writing—original draft. DVD: investigation and formal analysis. LVTS: validation and resources. TND: resources. LTN: investigation. HTHU: investigation.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vo Thang, N., Vu Thi, D., Ngo Thi My, B. et al. The synthesis of cubic Fe2O3·TiO2 material and its application in heterogeneous photo-Fenton degradation of dyes under visible light. J Nanopart Res 26, 22 (2024). https://doi.org/10.1007/s11051-024-05925-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05925-4