Abstract
Background
Andrographolide (AG) is a lactone diterpene with valuable biological activities. This in vitro study evaluated whether AG can protect cardiomyocytes under toxicities triggered with anti-cancer chemotherapeutic agents, doxorubicin (DOX) and arsenic trioxide (ATO).
Methods and results
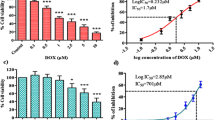
H9C2 cells were pretreated with AG (0.5–10 µM) for 24 h and then exposed to DOX (1 μM) or ATO (35 μM) for another 24 h period. For determination of cell viability or cytotoxicity, MTT and lactate dehydrogenase (LDH) assay were used. Total oxidant and antioxidant capacities were estimated by determining hydroperoxides and ferric reducing antioxidant power (FRAP) levels. Real time-polymerase chain reaction was also used for quantitative evaluation of TLR4 gene expression. AG inhibited cardiomyocytes proliferation at the concentrations of more than 20 μM. However, it considerably enhanced cell viability and decreased cytotoxicity of DOX and ATO at the concentration range of 2.5–10 μM in MTT and LDH assays. AG significantly declined hydroperoxides concentration in ATO-treated cardiomyocytes and raised FRAP value in DOX- and ATO-treated cells. Furthermore, AG notably lessened TLR4 expression in H9C2 cells after exposure to DOX- and ATO.
Conclusion
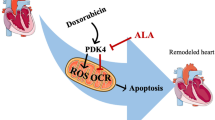
In conclusion, these data presented that AG was able to reverse DOX- and ATO-induced cardiotoxicity in vitro. The cardiomyocyte protective activities of AG may be due to the decrease in TLR4 expression and total oxidant capacity and increase in total antioxidant capacity.
Graphical Abstract






Similar content being viewed by others
References
Altena R, Perik PJ, van Veldhuisen DJ, de Vries EG, Gietema JA (2009) Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol 10:391–399. https://doi.org/10.1016/S1470-2045(09)70042-7
Bird BR, Swain SM (2008) Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res 14:14–24. https://doi.org/10.1158/1078-0432.CCR-07-1033
Farvadi F, Tamaddon AM, Abolmaali SS, Sobhani Z, Yousefi GH (2014) Micellar stabilized single-walled carbon nanotubes for a pH-sensitive delivery of doxorubicin. Res Pharm Sci 9:1–10
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229. https://doi.org/10.1124/pr.56.2.6
Chen B, Peng X, Pentassuglia L, Lim C, Sawyer DB (2007) Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol 7:114–121. https://doi.org/10.1007/s12012-007-0005-5
Akimoto H, Bruno NA, Slate DL, Biilingham ME, Torti SV, Torti FM (1993) Effect of verapamil on doxorubicin cardiotoxicity: altered muscle gene expression in cultured neonatal rat cardiomyocytes. Cancer Res 53:4658–4664
Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamrajv S (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem 234–235:119–124
Schlattner T, Zaugg M, Zuppinger M, Wallimann T, Schlattner U (2006) New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 41:389–405. https://doi.org/10.1016/j.yjmcc.2006.06.009
Tinajero J, El-Shami K, Wu X, Smith BD, Newman MJ (2022) Arsenic trioxide for acute promyelocytic leukemia in a patient on chronic hemodialysis. Leuk Res Rep 17:100304. https://doi.org/10.1016/j.lrr.2022.100304
Vineetha VP, Raghu KG (2019) An overview on arsenic trioxide-induced cardiotoxicity. Cardiovasc Toxicol 19:105–119. https://doi.org/10.1007/s12012-018-09504-7
Zhang JY, Sun GB, Wang M et al (2016) Arsenic trioxide triggered calcium homeostasis imbalance and induced endoplasmic reticulum stress-mediated apoptosis in adult rat ventricular myocytes. Toxicol Res 5:682–688. https://doi.org/10.1039/c5tx00463b
Sadat Alamolhodaei N, Shirani K, Karimi G (2015) Arsenic cardiotoxicity: an overview. Environ Toxicol Pharmacol 40:1005–1014. https://doi.org/10.1016/j.etap.2020.103524
Hossain MD, Urbi Z, Sule A, Rahman KM (2014) Andrographis paniculata (Burm. F.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J 2014:274905. https://doi.org/10.1155/2014/274905
Banerjee M, Chattopadhyay S, Choudhuri T et al (2016) Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J Biomed Sci 23:40. https://doi.org/10.1186/s12929-016-0257-0
Yang T, Shi HX, Wang ZT et al (2012) Hypolipidemic effects of andrographolide and neoandrographolide in mice and rats. Phytother Res 27:618–623. https://doi.org/10.1002/ptr.4771
Shi S, Ji X, Shi J et al (2022) Andrographolide in atherosclerosis: integrating network pharmacology and in vitro pharmacological evaluation. Biosci Rep. https://doi.org/10.1042/BSR20212812
Xie S, Deng W, Chen J et al (2020) Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci 16:12–26. https://doi.org/10.7150/ijbs.37269
Woo AY, Waye MM, Tsui SK, Yeung ST, Cheng CH (2008) Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J Pharmacol Exp Ther 325:226–235. https://doi.org/10.1124/jpet.107.133918
Gao H, Wang J (2016) Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF-κB signaling pathway. Mol Med Rep 13:1827–1832. https://doi.org/10.3892/mmr.2015.4703
Malekara E, Pazhouhi M, Rashidi I, Jalili C (2020) Anti-proliferative and cytotoxic effect of Iranian snake (Vipera raddei kurdistanica) venom on human breast cancer cells via reactive oxygen species-mediated apoptosis. Res Pharm Sci 15:76–86. https://doi.org/10.4103/1735-5362.278717
Feghhi-Najafabadi S, Safaeian L, Zolfaghari B (2019) In vitro antioxidant effects of different extracts obtained from the leaves and seeds of Allium ampeloprasum subsp. persicum. J Herbmed Pharmacol 8:256–260. https://doi.org/10.15171/jhp.2019.37
Safaeian L, Ghasemi-Dehkordi N, Javanmard SH, Namvar H (2015) Antihypertensive and antioxidant effects of a hydroalcoholic extract obtained from aerial parts of Otostegia persica (Burm.) Boiss. Res Pharm Sci 10:192–199
Safaeian L, Shafiee F, Naderi M (2022) Pramlintide: an amylin analogue protects endothelial cells against oxidative stress through regulating oxidative markers and NF-κb expression. Int J Prev Med 13:20. https://doi.org/10.4103/ijpvm.IJPVM_425_20
Kalam K, Marwick TH (2013) Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer 49:2900–2909. https://doi.org/10.1016/j.ejca.2013.04.030
Dey SK, Bose D, Hazra A (2013) Cytotoxic activity and apoptosis-inducing potential of di-spiropyrrolidino and di-spiropyrrolizidino oxindole andrographolide derivatives. PLoS ONE 8:e58055. https://doi.org/10.1371/journal.pone.0058055
Liang E, Liu X, Du Z (2018) Andrographolide ameliorates diabetic cardiomyopathy in mice by blockage of oxidative damage and NF-κB-mediated inflammation. Oxid Med Cell Longev 25(2018):9086747. https://doi.org/10.1155/2018/9086747
Wu QQ, Ni J, Zhang N, Yang R, Zhao Y (2017) Andrographolide protects against aortic banding-induced experimental cardiac hypertrophy by inhibiting MAPKs signaling. Front Pharmacol 8:808. https://doi.org/10.3389/fphar.2017.00808
Zhang J, Zhu D, Wang Y (2015) Andrographolide attenuates LPS-induced cardiac malfunctions through inhibition of IκB phosphorylation and apoptosis in mice. Cell Physiol Biochem 37:1619–1628. https://doi.org/10.1159/000438528
Mussard E, Cesaro A, Lespessailles E, Legrain B, Berteina-Raboin S, Toumi H (2019) Andrographolide, a natural antioxidant: an update. Antioxidants 8:571. https://doi.org/10.3390/antiox8120571
Riad A, Bien S, Gratz M et al (2008) Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart fail 10:233–243. https://doi.org/10.1016/j.ejheart.2008.01.004
El-Zayat SR, Sibaii H, Mannaa FA (2019) Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Cent 43:187. https://doi.org/10.1186/s42269-019-0227-2
Ma Y, Zhang X, Bao H et al (2012) Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin induced cardiomyopathy in mice. PLoS ONE 7:e40763. https://doi.org/10.1371/journal.pone.0040763
Zheng B, Yang Y, Li J et al (2021) Magnesium isoglycyrrhizinate alleviates arsenic trioxide-induced cardiotoxicity: contribution of Nrf2 and TLR4/NF-κB signaling pathway. Drug Des Devel Ther 15:543–556. https://doi.org/10.2147/DDDT.S296405
Nie X, Chen SR, Wang K et al (2017) Attenuation of innate immunity by andrographolide derivatives through NF-κB signaling pathway. Sci Rep 7:4738. https://doi.org/10.1038/s41598-017-04673-x
Zhang QQ, Ding Y, Lei Y et al (2014) Andrographolide suppress tumor growth by inhibiting TLR4/NF-κB signaling activation in insulinoma. Int J Biol Sci 10:404–414. https://doi.org/10.7150/ijbs.7723
Zhu T, Wang DX, Zhang W et al (2013) Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-κB. PLoS ONE 8(2):e56407. https://doi.org/10.1371/journal.pone.0056407
Ding Y, Chen L, Wu W, Yang J, Yang Z, Liu S (2017) Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-kappaB and JAK-STAT signaling pathway. Microbes Infect 19:605–615. https://doi.org/10.1016/j.micinf.2017.08.009
Chan SJ, Wong WS, Wong PT, Bian JS (2010) Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol 161:668–679. https://doi.org/10.1111/j.1476-5381.2010.00906.x
Chen YY, Hsu MJ, Hsieh CY, Lee LW, Chen ZC, Sheu JR (2014) Andrographolide inhibits nuclear factor-κB activation through JNK-Akt-p65 signaling cascade in tumor necrosis factor-α-stimulated vascular smooth muscle cells. Sci World J 2014:130381. https://doi.org/10.1155/2014/130381
Li Y, He S, Tang J et al (2017) Andrographolide inhibits inflammatory cytokines secretion in LPS-stimulated RAW2647 cells through suppression of NF-κB/MAPK signaling pathway. Evid Based Complement Alternat Med 2017:8248142. https://doi.org/10.1155/2017/8248142
Acknowledgements
This research was funded by Vice-Chancellery for Research and Technology of Isfahan University of Medical Sciences (Grant No. 299264).
Author information
Authors and Affiliations
Contributions
LS and FS contributed to the study conception and design. Material preparation, data collection and analysis was performed by SH. SH wrote the manuscript in consultation with LS and FS All authors discussed the results and approved the final form of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Disclosure
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safaeian, L., Shafiee, F. & Haghighatnazar, S. Andrographolide protects against doxorubicin-and arsenic trioxide-induced toxicity in cardiomyocytes. Mol Biol Rep 50, 389–397 (2023). https://doi.org/10.1007/s11033-022-08042-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08042-4