Abstract
Background/aim
The gradual accumulation of genetic and epigenetic alterations can lead to the development of colorectal cancer. In the last decade much research has been done to discover how methylation as an epigenetic alteration leads to carcinogenesis. While Methylation is a biological process, it can influence gene expression by affecting the promoter activity. This article reviews the role of methylation in critical pathways in CRC.
Methods
In this study using appropriate keywords, all research and review articles related to the role of methylation on different cancers were collected and analyzed. Also, existing information on methylation detection methods and therapeutic sensitivity or resistance due to DNA methylation were reviewed.
Results
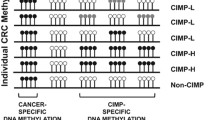
The results of this survey revealed that while Methylation is a biological process, it can influence gene expression by affecting the promoter activity. Promoter methylation is associated with up or downregulation of genes involved in critical pathways, including cell cycle, DNA repair, and cell adherence. Hence promoter methylation can be used as a molecular tool for early diagnosis, improving treatment, and predicting treatment resistance.
Conclusion
Current knowledge on potential methylation biomarkers for diagnosis and prognoses of CRC has also been discussed. Our survey proposes that a multi-biomarker panel is more efficient than a single biomarker in the early diagnosis of CRC.

Similar content being viewed by others
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Arnold M et al (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691
Imperiale TF et al (2014) Multitarget stool DNA testing for colorectal-cancer screening. New Engl J Med 370(14):1287–1297
Pino MS, Chung DC (2010) The chromosomal instability pathway in colon cancer. Gastroenterology 138(6):2059–2072
André T et al (2015) Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 33(35):4176–4187
Consortium, APG (2017) AACR project GENIE: powering precision medicine through aninternational consortium. Cancer Discov 7(8):818–831
Sjöblom T et al (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314(5797):268–274
Gargalionis AN et al (2012) Histone modifications as a pathogenic mechanism of colorectal tumorigenesis. Int J Biochem Cell Biol 44(8):1276–1289
Xie X et al (2016) Long non-coding RNAs in colorectal cancer. Oncotarget 7(5):5226
Luo Y et al (2014) Differences in DNA methylation signatures reveal multiple pathways of progression from adenoma to colorectal cancer. Gastroenterology 147(2):418–429
Greger V et al (1989) Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet 83(2):155–158
Kim MS, Lee J, Sidransky D (2010) DNA methylation markers in colorectal cancer. Cancer Metastasis Rev 29(1):181–206
Ng JM-K, Yu J (2015) Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci 16(2):2472–2496
Kang X-C et al (2016) Promoter methylation and expression of SOCS-1 affect clinical outcome and epithelial-mesenchymal transition in colorectal cancer. Biomed Pharmacother 80:23–29
Koinuma K et al (2006) Epigenetic silencing of AXIN2 in colorectal carcinoma with microsatellite instability. Oncogene 25(1):139–146
Sun J et al (2019) The role of m SEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC Cancer 19(1):450
Rokni P et al (2018) BMP3 promoter hypermethylation in plasma-derived cell-free DNA in colorectal cancer patients. Genes Genomics 40(4):423–428
Draht MX et al (2018) Prognostic DNA methylation markers for sporadic colorectal cancer: a systematic review. Clin Epigenet 10(1):35
Grady W et al (2008) Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene 27(27):3880–3888
Jing N et al (2017) A meta-analysis of the TFPI2 hyper-methylation frequency and colorectal cancer risk. Biomed Res 28(2):951–956
Huang Q et al (2017) Screening of exon methylation biomarkers for colorectal cancer via LC-MS/MS strategy. J Mass Spectrom 52(12):860–866
Chen X et al (2019) DNA methylation-regulated and tumor‐suppressive roles of miR‐487b in colorectal cancer via targeting MYC, SUZ12, and KRAS. Cancer Med 8(4):1694–1709
Han YD et al (2019) Early detection of colorectal cancer based on presence of methylated syndecan-2 (SDC2) in stool DNA. Clin Epigenet 11(1):51
Rayess H, Wang MB, Srivatsan ES (2012) Cellular senescence and tumor suppressor gene p16. Int J Cancer 130(8):1715–1725
Hsu C-H et al (2020) Multiple gene promoter methylation and clinical stage in adjacent normal tissues: effect on prognosis of colorectal cancer in Taiwan. Sci Rep 10(1):1–11
Liang J-T et al (1999) Hypermethylation of the p16 gene in sporadic T3N0M0 stage colorectal cancers: association with DNA replication error and shorter survival. Oncology 57(2):149–156
Dasgupta N et al (2015) Caveolin-1 is transcribed from a hypermethylated promoter to mediate colonocyte differentiation and apoptosis. Exp Cell Res 334(2):323–336
Torrejón B et al (2017) Caveolin-1 is markedly downregulated in patients with early-stage colorectal cancer. World J Surg 41(10):2625–2630
Yu X et al (2005) Chfr is required for tumor suppression and aurora A regulation. Nat Genet 37(4):401–406
Bertholon J et al (2003) Chfr inactivation is not associated to chromosomal instability in colon cancers. Oncogene 22(55):8956–8960
Morioka Y et al (2006) Aberrant methylation of the CHFR gene is frequently detected in non-invasive colorectal cancer. Anticancer Res 26(6B):4267–4270
Sun Z et al (2017) The diagnostic and prognostic value of CHFR hypermethylation in colorectal cancer, a meta-analysis and literature review. Oncotarget 8(51):89142
Pelosof L et al (2014) CHFR silencing or microsatellite instability is associated with increased antitumor activity of docetaxel or gemcitabine in colorectal cancer. Int J Cancer 134(3):596–605
Matthaios D et al (2016) Methylation status of the APC and RASSF1A promoter in cellfree circulating DNA and its prognostic role in patients with colorectal cancer. Oncol Lett 12(1):748–756
Liang T-J et al (2017) APC hypermethylation for early diagnosis of colorectal cancer: a meta-analysis and literature review. Oncotarget 8(28):46468
Liu X et al (2019) DNA methylation of SFRP1, SFRP2, and WIF1 and prognosis of postoperative colorectal cancer patients. BMC Cancer 19(1):1212
Wang Z et al (2018) Effects of secreted frizzled-related protein 1 on proliferation, migration, invasion, and apoptosis of colorectal cancer cells. Cancer Cell Int 18(1):1–10
Kumar A et al (2019) Prognostic relevance of SFRP1 gene promoter methylation in colorectal carcinoma. Asian Pac J Cancer Prev 20(5):1571
Satelli A, Rao US (2011) Galectin-1 is silenced by promoter hypermethylation and its re-expression induces apoptosis in human colorectal cancer cells. Cancer Lett 301(1):38–46
Katzenmaier EM et al (2017) Analyzing epigenetic control of galectin expression indicates silencing of galectin-12 by promoter methylation in colorectal cancer. Iubmb Life 69(12):962–970
Zhang Y et al (2014) Bone morphogenetic protein 2 inhibits the proliferation and growth of human colorectal cancer cells. Oncol Rep 32(3):1013–1020
Miura T et al (2020) Methylation of bone morphogenetic protein 2 is associated with poor prognosis in colorectal cancer. Oncol Lett 19(1):229–238
Naini MA et al (2018) O6-methyguanine-DNA methyl transferase (MGMT) promoter methylation in serum DNA of iranian patients with colorectal cancer. Asian Pac J Cancer Prev 19(5):1223
Niv Y (2007) Microsatellite instability and MLH1 promoter hypermethylation in colorectal cancer. World J Gastroenterol 13(12):1767
Kawasaki T et al (2008) WRN promoter methylation possibly connects mucinous differentiation, microsatellite instability and CpG island methylator phenotype in colorectal cancer. Mod Pathol 21(2):150–158
Kerachian MA et al (2019) Epigenetic inactivation of protocadherin 10 by methylation in colorectal cancer. Acta Med Iran 67(8):472–477
Xu Z et al (2015) Fibulin-1 is downregulated through promoter hypermethylation in colorectal cancer: a CONSORT study. Medicine 94(13):e663. https://doi.org/10.1097/MD.0000000000000663
Chen K et al (2011) CADM1/TSLC1 inactivation by promoter hypermethylation is a frequent event in colorectal carcinogenesis and correlates with late stages of the disease. Int J Cancer 128(2):266–273
Yuan S et al (2019) Cadherin-11 is inactivated due to promoter methylation and functions in colorectal cancer as a tumour suppressor. Cancer Manag Res 11:2517
Neumeyer S et al (2019) Genome-wide D.N.A. methylation differences according to oestrogen receptor beta status in colorectal cancer. Epigenetics 14(5):477–493
Xia T et al (2019) Androgen receptor gene methylation related to colorectal cancer risk. Endocr Connect 8(7):979–987
Shademan M et al (2020) Promoter methylation, transcription, and retrotransposition of LINE-1 in colorectal adenomas and adenocarcinomas. Cancer Cell Int 20(1):1–6
Kato K et al (2009) DNA hypomethylation at the CpG island is involved in aberrant expression of the L1 cell adhesion molecule gene in colorectal cancer. Int J Oncol 35(3):467–476
Dimberg J et al (2012) DNA promoter methylation status and protein expression of interleukin-8 in human colorectal adenocarcinomas. Int J Colorectal Dis 27(6):709–714
Ying X et al (2019) Significant association of EED. promoter hypomethylation with colorectal cancer. Oncol Lett 18(2):1564–1570
Locke WJ et al (2019) DNA methylation cancer biomarkers: translation to the clinic. Front Genet 10:1150. https://doi.org/10.3389/fgene.2019.01150
Freitas M et al (2018) A novel DNA methylation panel accurately detects colorectal cancer independently of molecular pathway. J Trans Med 16(1):45
Lidgard GP et al (2013) Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol 11(10):1313–1318
Chang E et al (2010) Detection of colorectal neoplasm using promoter methylation of ITGA4, SFRP2, and p16 in stool samples: a preliminary report in Korean patients. Hepato-gastroenterology 57(101):720
Hu H et al (2018) Diagnostic value of WIF1 methylation for colorectal cancer: a meta-analysis. Oncotarget 9(4):5378
Lee BB et al (2009) Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res 15(19):6185–6191
Jensen S et al (2019) Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer—a clinical biomarker discovery and validation study. Clin Epigenet 11(1):1–14
Song Y-C et al (2014) Detection of miR-34a and miR-34b/c in stool sample as potential screening biomarkers for noninvasive diagnosis of colorectal cancer. Med Oncol 31(4):894
Da Silva T et al (2015) P-192 DNA methylation profile of APC and DKK2 genes as biomarkers in colorectal cancer patients. Ann Oncol 26:iv55
Ahlquist DA et al (2012) Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology 142(2):248–256
Wu D et al (2016) Detection of colorectal cancer using a simplified SEPT9 gene methylation assay is a reliable method for opportunistic screening. J Mol Diagn 18(4):535–545
Barták BK et al (2017) Colorectal adenoma and cancer detection based on altered methylation pattern of SFRP1, SFRP2, SDC2, and PRIMA1 in plasma samples. Epigenetics 12(9):751–763
Müller HM et al (2004) Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet 363(9417):1283–1285
Zhang W et al (2007) DNA stool test for colorectal cancer: hypermethylation of the secreted frizzled-related protein-1 gene. Dis Colon Rectum 50(10):1618–1627
Li W-H et al (2015) Detection of SNCA and FBN1 methylation in the stool as a biomarker for colorectal cancer. Dis Markers 2015:657570. https://doi.org/10.1155/2015/657570
Chen J et al (2019) DNA methylation biomarkers in stool for early screening of colorectal cancer. J Cancer 10(21):5264
Hellebrekers DM et al (2009) GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res 15(12):3990–3997
Jin P et al (2015) Performance of a second-generation methylated SEPT9 test in detecting colorectal neoplasm. J Gastroenterol Hepatol 30(5):830–833
Zhao G et al (2020) Aberrant DNA methylation of SEPT9 and SDC2 in stool specimens as an integrated biomarker for colorectal cancer early detection. Front Genet 11:643
Chen Y et al (2019) Performance of a novel blood-based early colorectal cancer screening assay in remaining serum after the blood biochemical test. Dis Markers 2019:5232780. https://doi.org/10.1155/2019/5232780
Wang Y et al (2017) Methylation of ZNF331 is an independent prognostic marker of colorectal cancer and promotes colorectal cancer growth. Clin Epigenet 9(1):115
Chung H-H et al (2019) A novel prognostic DNA methylation panel for colorectal cancer. Int J Mol Sci 20(19):4672
Heitzer E et al (2012) P-0198 identification of prognostic methylation markers in patients with early stage colorectal cancer. Ann Oncol 23:iv87
Wallner M et al (2006) Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res 12(24):7347–7352
Nilsson TK, Löf-Öhlin ZM, Sun X-F (2013) DNA methylation of the p14ARF, RASSF1A and APC1A genes as an independent prognostic factor in colorectal cancer patients. Int J Oncol 42(1):127–133
Barros-Silva D et al (2018) Profiling DNA methylation based on next-generation sequencing approaches: new insights and clinical applications. Genes 9(9):429
Heyn H, Esteller M (2012) DNA methylation profiling in the clinic: applications and challenges. Nat Rev Genet 13(10):679–692
Li P et al (2013) An integrated workflow for DNA methylation analysis. J Genet Genomics 40(5):249–260
Trinh BN, Long TI, Laird PW (2001) DNA methylation analysis by MethyLight technology. Methods 25(4):456–462
Nygren AO et al (2005) Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res 33(14):e128–e128
Chen J-J, Wang A-Q, Chen Q-Q (2017) DNA methylation assay for colorectal carcinoma. Cancer Biol Med 14(1):42
Melka MG et al (2014) The effects of olanzapine on genome-wide DNA methylation in the hippocampus and cerebellum. Clin Epigenet 6(1):1
Swathy B, Banerjee M (2017) Pharmaco-epigenomic response of antipsychotic drugs in theranostics of schizophrenia. Eur Neuropsychopharmacol 27:S404–S405
Guidotti A, Grayson DR (2014) DNA methylation and demethylation as targets for antipsychotic therapy. Dialog Clin Neurosci 16(3):419
Nakamura K et al (2015) DNA methyltransferase inhibitor zebularine induces human cholangiocarcinoma cell death through alteration of DNA methylation status. PLoS One 10(3):e0120545
Gailhouste L et al (2018) Epigenetic reprogramming using 5-azacytidine promotes an anti-cancer response in pancreatic adenocarcinoma cells. Cell Death Dis 9(5):1–12
Ramakrishnan S et al (2017) Decitabine, a DNA-demethylating agent, promotes differentiation via NOTCH1 signaling and alters immune-related pathways in muscle-invasive bladder cancer. Cell Death Dis 8(12):1–13
Yaffee P et al (2015) Review of systemic therapies for locally advanced and metastatic rectal cancer. J Gastrointest Oncol 6(2):185
Van der Jeught K et al (2018) Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 24(34):3834
Humeniuk R et al (2009) Epigenetic reversal of acquired resistance to 5-fluorouracil treatment. Mol Cancer Ther 8(5):1045–1054 https://doi.org/10.1158/1535-7163.MCT-08-0717
Baharudin R et al (2017) Identification of predictive DNA methylation biomarkers for chemotherapy response in colorectal cancer. Front Pharmacol 8:47
Cha Y et al (2019) Association of CHFR promoter methylation with treatment outcomes of irinotecan-based chemotherapy in metastatic colorectal cancer. Neoplasia 21(1):146–155
Sun X et al (2017) Promoter methylation of RASSF1A indicates prognosis for patients with stage II and III colorectal cancer treated with oxaliplatin-based chemotherapy. Med Sci Monitor 23:5389
He T et al (2017) Methylation of SLFN11 is a marker of poor prognosis and cisplatin resistance in colorectal cancer. Epigenomics 9(6):849–862
Pelosof L et al (2017) GPX3 promoter methylation predicts platinum sensitivity in colorectal cancer. Epigenetics 12(7):540–550
Acknowledgements
This work was supported by a Grant (No. 69944) from Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Not applicable.
Research involving human participants and/or animals
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghadiri Moghaddam, F., Farajnia, S., Karbalaei-Mahdi, M. et al. Epigenetic insights in the diagnosis, prognosis, and treatment selection in CRC, an updated review. Mol Biol Rep 49, 10013–10022 (2022). https://doi.org/10.1007/s11033-022-07569-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07569-w