Abstract
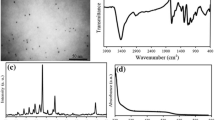
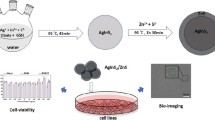
Near-infrared quantum dots (NIR QDs) are promising candidate for the fluorescent probes due to their better penetration depth, long-lived luminescence with size-tunable photoluminescence wavelengths. Glutathione-coated silver sulfide quantum dots (GSH-Ag2S QDs) were synthesized using AgNO3 and Na2S in the aqueous media and they can give reaction with glutathione reductase (GR) and glutathione-s transferase (GST) enzymes as acting substrate analogue in vitro. Investigation of the toxicity of the nanomaterials are necessary to use them in the medical field and biomedical applications. Thus, in this study we investigated biocompatibility of the GSH-Ag2S QDs in vitro using 293 T and CFPAC-1 cell lines. Cell viability by MTT assay, light microscopy, fluorescence microscopy, oxidative stress enzyme activities and ICP-MS analysis were performed to evaluate the cytotoxicity and internalization of the GSH-Ag2S QDs. GSH-Ag2S QDs showed great biocompatibility with both cell lines and did not cause imbalance in the oxidative stress metabolism. The ultralow solubility product constant of Ag2S QDs (Ksp = 6.3 × 10–50) prevents release of Ag ions into the biological systems that is in agreement with data obtained by ICP-MS. In conclusion, this data prove potential of GSH-Ag2S QDs as a biocompatible optical probe to be used for the detection and/or targeting of GSH impaired diseases including cancer.
Graphic abstract










Similar content being viewed by others
Data availability
The data obtained/analyzed during this study are available from the corresponding author on reasonable request.
References
Duman FD, Hocaoglu I, Ozturk DG, Gozuacik D, Kiraz A, Yagci Acar H (2012) Development of highly luminescent and cytocompatible near-IR-emitting aqueous Ag2S quantum dots. J Mater Chem 7:11352–11362. https://doi.org/10.1039/c2jm31959d
Hocaoglu I, Asik D, Ulusoy G, Grandfils C, Ojea-Jimenez I, Rossi F, Kiraz A, Doğan N, Acar HY (2015) Cyto/hemocompatible magnetic hybrid nanoparticles (Ag2S-Fe3O4) with luminescence in the near-infrared region as promising theranostic materials. Colloids Surf B Biointerfaces 133:198–207. https://doi.org/10.1016/j.colsurfb.2015.05.051
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 9:763–775. https://doi.org/10.1038/nmeth.1248
Chinnathambi S, Shirahata N (2019) Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci Technol Adv Mater 20:337–355. https://doi.org/10.1080/14686996.2019.1590731
Nakane Y, Tsukasaki Y, Sakata T, Yasuda H, Jin T (2013) Aqueous synthesis of glutathione-coated PbS quantum dots with tunable emission for non-invasive fluorescence imaging in the second near-infrared biological window (1000–1400 nm). Chem Commun 49:7584–7586. https://doi.org/10.1039/c3cc44000a
Xue B, Deng DW, Cao J, Liu F, Li X, Akers W, Achilefu S, Gu YQ (2012) Synthesis of NAC capped near infrared-emitting CdTeS alloyed quantum dots and application for in vivo early tumor imaging. Dalt Trans 41:4935–4947. https://doi.org/10.1039/c2dt12436j
Tiwari DK, Jin T, Behari J (2011) Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol Mech Methods 1:13–24. https://doi.org/10.3109/15376516.2010.529184
Pérez E, Olmo R, Teijón C, Muñíz E, Montero N, Teijón JM, Blanco MD (2015) Biocompatibility evaluation of pH and glutathione-responsive nanohydrogels after intravenous administration. Colloids Surf B Biointerfaces 136:222–231. https://doi.org/10.1016/j.colsurfb.2015.09.017
Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC (2012) Interactions of nanomaterials and biological systems: implications to personalized nanomedicine. Adv Drug Deliv Rev 64:1363–1384. https://doi.org/10.1016/j.addr.2012.08.005
Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P (2013) Biocompatibility of engineered nanoparticles for drug delivery. J Control Release 166:182–194. https://doi.org/10.1016/j.jconrel.2012.12.013
Sivasankaran U, Jesny S, Jose AR, Girish Kumar K (2017) Fluorescence determination of glutathione using tissue paper-derived carbon dots as fluorophores. Anal Sci 33:281–285. https://doi.org/10.2116/analsci.33.281
Aydemir D, Hashemkhani M, Durmusoglu EG, Acar HY, Ulusu NN (2019) A new substrate for glutathione reductase: glutathione coated Ag2S quantum dots. Talanta 194:501–506. https://doi.org/10.1016/j.talanta.2018.10.049
Aydemir D, Hashemkhani M, Acar HY, Ulusu NN (2019) In vitro interaction of glutathione S-transferase-pi enzyme with glutathione-coated silver sulfide quantum dots: a novel method for biodetection of glutathione S-transferase enzyme. Chem Biol Drug Des 94:2094–2102. https://doi.org/10.1111/cbdd.13614
Du Y, Xu B, Fu T, Cai M, Li F, Zhang Y, Wang Q (2010) Near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J Am Chem Soc 132:1470–1471
Zhang Y, Hong G, Zhang Y, Chen G, Li F, Dai H, Wang Q (2012) Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infra red window. ACS Nano 6:3695–3702. https://doi.org/10.1021/nn301218z
Hong G, Robinson JT, Zhang Y, Diao S, Antaris AL, Wang Q, Dai H (2012) In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew Chem Int Ed Engl 51:9818–9821. https://doi.org/10.1002/anie.201206059
Zhang Y, Zhang Y, Hong G, He W, Zhou K, Yang K, Li F, Chen G, Liu Z, Dai H, Wang Q (2013) Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice. Biomaterials 34:3639–3646. https://doi.org/10.1016/j.biomaterials.2013.01.089
Hu F, Li C, Zhang Y, Wang M, Wu D, Wanget D (2015) Real-time in vivo visualization of tumor therapy by a near-infrared-II Ag2S quantum dot-based theranostic nanoplatform. Nano Res 8:1637–1647. https://doi.org/10.1007/s12274-014-0653-2
Hashemkhani M, Bilici K, Muti A, Sennaroglu A, Yagci-Acar H (2020) Ag2S-glutathione quantum dots for NIR image guided photothermal therapy. New J Chem. https://doi.org/10.1039/C9NJ04608A
Lv H, Zhen C, Liu J, Yang P, Hu L, Shang P (2019) Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid Med Cell Longev. https://doi.org/10.1155/2019/3150145
Buchman JT, Hudson-Smith NV, Landy KM, Haynes CL (2019) Understanding nanoparticle toxicity mechanisms to inform redesign strategies to reduce environmental impact. Acc Chem Res 52:1632–1642. https://doi.org/10.1021/acs.accounts.9b00053
Aydemir D, Karabulut G, Şimşek G, Gok M, Barlas N, Ulusu NN (2018) Impact of the di(2-ethylhexyl) phthalate administration on trace element and mineral levels in relation of kidney and liver damage in rats. Biol Trace Elem Res 186:474–488. https://doi.org/10.1007/s12011-018-1331-0
Aydemir D, Simsek G, Ulusu NN (2020) Dataset of the analyzing trace elements and minerals via ICP-MS: method validation for the mammalian tissue and serum samples. Data Brief 29:105218. https://doi.org/10.1016/j.dib.2020.105218
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976. https://doi.org/10.1016/j.envint.2006.06.014
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531. https://doi.org/10.1016/j.envint.2010.10.012
Lai L, Li SJ, Feng J, Mei P, Ren ZH, Chang YL, Liu Y (2017) Effects of surface charges on the bactericide activity of cdte/zns quantum dots: a cell membrane disruption perspective. Langmuir 33:2378–2386. https://doi.org/10.1021/acs.langmuir.7b00173
Williams DN, Pramanik S, Brown RP, Zhi B, McIntire E, Hudson-Smith NV, Haynes CL, Rosenzweig Z (2018) Adverse interactions of luminescent semiconductor quantum dots with liposomes and Shewanella oneidensis. ACS Appl Nano Mater 1:4788–4800. https://doi.org/10.1021/acsanm.8b01000
Imlay JA (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. https://doi.org/10.1038/nrmicro3032
Hardman R (2006) A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Health Perspect 114:165–172. https://doi.org/10.1289/ehp.8284
Jiang Y, Dong Y, Luo Q, Li N, Wu G, Gao H (2014) Protection from oxidative stress relies mainly on derepression of OxyR-dependent KatB and Dps in Shewanella oneidensis. J Bacteriol 196:445–458. https://doi.org/10.1128/JB.01077-13
Liu N, Tang M (2019) Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J Appl Toxicol 40:16–36. https://doi.org/10.1002/jat.381
Singh BR, Singh BN, Khan W, Singh HB, Naqvi AH (2012) ROS-mediated apoptotic cell death in prostate cancer LNCaP cells induced by biosurfactant stabilized CdS quantum dots. Biomaterials 33:5753–5767. https://doi.org/10.1016/j.biomaterials.2012.04.045
Wang J, Liu R, Liu B (2016) Cadmium-containing quantum dots: Current perspectives on their application as nanomedicine and toxicity concerns. Mini Rev Med Chem 16:905–916. https://doi.org/10.2174/1389557516666160211121247
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627. https://doi.org/10.1126/science.1114397
Tandogan B, Ulusu NN (2010) A comparative study with colchicine on glutathione reductase. Protein J 29:380–385. https://doi.org/10.1007/s10930-010-9263-3
Tandogan B, Kuruüzüm-Uz A, Sengezer C, Güvenalp Z, Demirezer LÖ, Ulusu NN (2011) In vitro effects of rosmarinic acid on glutathione reductase and glucose 6-phosphate dehydrogenase. Pharm Biol 49:587–594. https://doi.org/10.3109/13880209.2010.533187
Aydemir D, Ulusu NN (2020) Comment on the: molecular mechanism of CAT and SOD activity change under MPA-CdTe quantum dots induced oxidative stress in the mouse primary hepatocytes. Spectrochim Acta A 229:117792. https://doi.org/10.1016/j.saa.2019.117792
Aydemir D, Oztasci B, Barlas N, Ulusu NN (2019) Effects of butylparaben on antioxidant enzyme activities and histopathological changes in rat tissues. Arh Hig Rada Toksikol 70:315–324
Ulusu NN (2015) Glucose-6-phosphate dehydrogenase deficiency and Alzheimer’s disease: partners in crime? Hypothes Med Hypothes 85:219–223. https://doi.org/10.1016/j.mehy.2015.05.006
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336. https://doi.org/10.1016/0891-5849(94)00159-h
Hoshino N, Kimura T, Yamaji A, Ando T (1999) Damage to the cytoplasmic membrane of escherichia coli by catechin-copper (II) complexes. Free Radic Biol Med 27:1245–1250. https://doi.org/10.1016/s0891-5849(99)00157-4
Winterbourn CC (1995) Toxicity of iron and hydrogen peroxide: the fenton reaction. Toxicol Lett 82:969–974. https://doi.org/10.1016/0378-4274(95)03532-x
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200. https://doi.org/10.1007/s00204-013-1079-4
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871. https://doi.org/10.3390/ma5122850
Xie C, Zhang J, Ma Y, Ding Y, Zhang P, Zheng L, Chai Z, Zhao Y, Zhang Z, He X (2019) Bacillus subtilis causes dissolution of ceria nanoparticles at the nano–bio interface. Environ Sci Nano 6:216–223. https://doi.org/10.1039/c8en01002a
Naatz H, Lin S, Li R, Jiang W, Ji Z, Chang CH, Köser J, Thöming J, Xia T, Nel AE, Mädler L, Pokhrel S (2017) Safe-by- design of CuO nanoparticles via Fe-doping, Cu-O bond lengths variation, and biological assessment in cells and zebrafish embryos. ACS Nano 11:501–515. https://doi.org/10.1021/acsnano.6b06495
Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G (2016) Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine 12:789–799. https://doi.org/10.1016/j.nano.2015.11.016
Gunsolus IL, Mousavi MP, Hussein K, Bühlmann P, Haynes CL (2015) Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity. Environ Sci Technol 49:8078–8086. https://doi.org/10.1021/acs.est.5b01496
Mousavi MP, Gunsolus IL, Pérez De Jesús CE, Lancaster M, Hussein K, Haynes CL, Bühlmann P (2015) Dynamic silver speciation as studied with fluorous-phase ion-selective electrodes: effect of natural organic matter on the toxicity and speciation of silver. Sci Total Environ 537:453–461. https://doi.org/10.1016/j.scitotenv.2015.07.151
Hudson-Smith NV, Clement PL, Brown RP, Krause MOP, Pedersen JA, Haynes CL (2016) Research highlights: speciation and transformations of silver released from Ag NPs in three species. Environ Sci Nano 3:1236–1240. https://doi.org/10.1039/c6en90025a
Depaoli MR, Bischof H, Eroglu E, Burgstaller S, Ramadani-Muja J, Rauter T, Schinagl M, Waldeck-Weiermair M, Hay JC, Graier WF, Malli R (2019) Live cell imaging of signaling and metabolic activities. Pharmacol Ther 202:98–119. https://doi.org/10.1016/j.pharmthera.2019.06.003
Terry J (1994) The major electrolytes: sodium, potassium, and chloride. J Intraven Nurs 17:240–247
Page MJ, Di Cera E (2006) Role of Na + and K + in enzyme function. Physiol Rev 86:1049–1092. https://doi.org/10.1152/physrev.00008.2006
Palmer BF (2015) Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10:1050–1060. https://doi.org/10.2215/CJN.08580813
Palmer BF, Clegg DJ (2016) Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 40:480–490. https://doi.org/10.1152/advan.00121.2016
Brodie C, Bak A, Shainberg A, Sampson SR (1987) Role of Na-K ATPase in regulation of resting membrane potential of cultured rat skeletal myotubes. J Cell Physiol 130:191–198. https://doi.org/10.1002/jcp.1041300204
Ghasemi M, Khodaei N, Salari S, Eliassi A, Saghiri R (2014) Gating behavior of endoplasmic reticulum potassium channels of rat hepatocytes in diabetes. Iran Biomed J 18:165–172. https://doi.org/10.6091/ibj.1308.2014
Szewczyk A, Jarmuszkiewicz W, Kunz WS (2009) Mitochondrial potassium channels. IUBMB Life 61:134–143. https://doi.org/10.18388/pb.2018_132
Fletcher A (2011) Action potential: generation and propagation. Anaesth Intens Care 12:258–262. https://doi.org/10.1016/j.mpaic.2011.03.010
Kairane C, Mahlapuu R, Ehrlich K, Zilmer M, Soomets U (2014) The effects of different antioxidants on the activity of cerebrocortical MnSOD and Na, K-ATPase from post mortem Alzheimer's disease and age-matched normal brains. Curr Alzheimer Res 11:79–85. https://doi.org/10.2174/15672050113106660179
Acknowledgement
The authors gratefully acknowledge use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM) (252-172), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
Author information
Authors and Affiliations
Contributions
NNU and HYA conceived and designed the experiments. DA and MH supported the organization of the project, performed the experiments and analyzed the data. DA and MH wrote the paper. All contributing authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aydemir, D., Hashemkhani, M., Acar, H.Y. et al. Evaluation of the biocompatibility of the GSH-coated Ag2S quantum dots in vitro: a perfect example for the non-toxic optical probes. Mol Biol Rep 47, 4117–4129 (2020). https://doi.org/10.1007/s11033-020-05522-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05522-3