Abstract
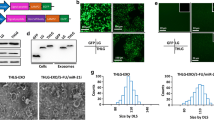
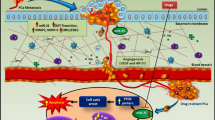
Prostate cancer (PCa) is a prevalent malignant neoplasm affecting the male reproductive system globally. However, the diagnostic and therapeutic approaches fall short of meeting the demands posed by PCa. Poor expression of miRNA-203 (miR-203) within PCa tissues and cells implies its potential utility as a diagnostic indicator for PCa. Exosomes (Exo), membranous vesicles released by various cells, are rich reservoirs of miRNAs. However, the presence of miR-203 presents within Exo derived from PCa cells remains unclarified. In this study, Exo was isolated from urine specimens collected from clinical PCa patients and LNCaP cells to detect miR-203 expression. Meanwhile, the impact of overexpressed miR-203 on M0 macrophages (mø) was analyzed. Subsequently, alterations in the proliferative, migratory, and invasive capacities of LNCaP cells were examined within a co-culture system featuring elevated miR-203 levels in both macrophages and LNCaP cells. Furthermore, the repercussions of miR-203 upregulation or inhibition were explored in a murine PCa tumor model. The results revealed that Exo manifested a circular or elliptical morphology, encapsulating a phospholipid bilayer approximately 100 nm in diameter. Notably, Exo readily infiltrated, with both Exo and miR-203-overexpressing Exo prompting macrophage polarization toward the M1 subtype. In the co-culture system, miR-203 exhibited pronounced suppression of LNCaP cell proliferation, migration, and invasion, while concurrently fostering apoptosis as compared with the LNCaP group (Control). In vivo experiments further disclosed that miR-203 greatly inhibited the growth of PCa tumors in nude mice. Markedly heightened expression of M1 macrophage markers such as IL-1β, IL-6, IL-12, CXCL9, and CXCL10 was observed within the tumor microenvironment following miR-203 intervention, as opposed to the model group. However, the introduction of miR-203 antagomir led to a reversal in tumor growth trends. This investigation indicates the presence of miR-203 within the urine of PCa patients and Exo originating from cells, and that miR-203 exerted antitumor effect by facilitating M1 macrophage polarization. Our study furnishes valuable insights into the potential applicability of miR-203 as a diagnostic biomarker and therapeutic target for PCa.






Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Bo B, Guo JB, Liu LR, Wei Q (2021) Mechanisms of drug resistance in endocrinotherapy for castration-resistant prostate cancer. Zhonghua Nan Ke Xue 27(2):167–171
Chang CC, Lee YC, Tsai HW et al (2015) Diagnostic role of serum free-to-total prostate specific antigen (PSA) ratio in prostate cancer with serum total concentration of PSA below 4 ng/mL. Asian Pac J Cancer Prev 16(13):5261–5264
Koppers-Lalic D, Hackenberg M, de Menezes R et al (2016) Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget 7(16):22566–22578
Bianchi L, Borghesi M, Schiavina R et al (2020) Predictive accuracy and clinical benefit of a nomogram aimed to predict (68)Ga-PSMA PET/CT positivity in patients with prostate cancer recurrence and PSA < 1 ng/ml external validation on a single institution database. Eur J Nucl Med Mol Imaging 47(9):2100–2105
Etzioni R, Penson DF, Legler JM et al (2002) Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 94(13):981–90
Iremashvili V, Pelaez L, Manoharan M et al (2012) Pathologic prostate cancer characteristics in patients eligible for active surveillance: a head-to-head comparison of contemporary protocols. Eur Urol 62(3):462–468
Sun Y (2016) Tumor microenvironment and cancer therapy resistance. Cancer Lett 380(1):205–215
Nabariya DK, Pallu R, Yenuganti VR (2020) Exosomes: the protagonists in the tale of colorectal cancer? Biochim Biophys Acta Rev Cancer 1874(2):188426
Dai J, Escara-Wilke J, Keller JM et al (2019) Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med 216(12):2883–2899
Tamkovich S, Tutanov O, Efimenko A et al (2019) Blood circulating exosomes contain distinguishable fractions of free and cell-surface-associated vesicles. Curr Mol Med 19(4):273–285
Pardini B, Cordero F, Naccarati A et al (2018) microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget 9(29):20658–20669
Wu D, Yan J, Shen X et al (2019) Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat Commun 10(1):3854
Skotland T, Ekroos K, Kauhanen D et al (2017) Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer 70:122–132
Stoorvogel W (2012) Functional transfer of microRNA by exosomes. Blood 119(3):646–648
Fujita K, Nonomura N (2018) Urinary biomarkers of prostate cancer. Int J Urol 25(9):770–779
Valentino A, Reclusa P, Sirera R et al (2017) Exosomal microRNAs in liquid biopsies: future biomarkers for prostate cancer. Clin Transl Oncol 19(6):651–657
Zhang LS, Ma HG, Sun FH et al (2019) MiR-203 inhibits the malignant behavior of prostate cancer cells by targeting RGS17. Eur Rev Med Pharmacol Sci 23(13):5667–5674
Yin XZ, Zhao DM, Zhang GX, Liu L (2016) Effect of miRNA-203 on cervical cancer cells and its underlying mechanism. Genet Mol Res 15(3):1–7
Deng B, Wang B, Fang J et al (2016) MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Sci Rep 6:28301
Yang L, Liang H, Wang Y et al (2016) MiRNA-203 suppresses tumor cell proliferation, migration and invasion by targeting Slug in gastric cancer. Protein Cell 7(5):383–387
Kim JS, Choi DW, Kim CS et al (2018) microRNA-203 induces apoptosis by targeting Bmi-1 in YD-38 oral cancer cells. Anticancer Res 38(6):3477–3485
Zhao S, Yao D, Chen J, Ding N (2013) Circulating miRNA-20a and miRNA-203 for screening lymph node metastasis in early stage cervical cancer. Genet Test Mol Biomark 17(8):631–636
Svennevig JL, Svaar H (1979) Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer 24(6):754–758
Shrivastava P, Singh SM, Singh N (2004) Effect of thymosin alpha 1 on the antitumor activity of tumor-associated macrophage-derived dendritic cells. J Biomed Sci 11(5):623–630
Domínguez-Soto A, Sierra-Filardi E, Puig-Kröger A et al (2011) Dendritic cell-specific ICAM-3-grabbing nonintegrin expression on M2-polarized and tumor-associated macrophages is macrophage-CSF dependent and enhanced by tumor-derived IL-6 and IL-10. J Immunol 186(4):2192–2200
Yuan R, Li S, Geng H et al (2017) Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int Immunopharmacol 49:30–37
Ding P, Wang W, Wang J et al (2014) Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem Biophys 70(3):1625–1631
Jenkins SJ, Ruckerl D, Cook PC et al (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332(6035):1284–1288
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795
Genin M, Clement F, Fattaccioli A et al (2015) M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15:577
Acknowledgements
We are particularly grateful to all the people who have given us help on our article.
Funding
This research was supported by the General Project of Wuxi Municipal Health Commission (Project No. M202155).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: Lian-Sheng Zhang, Qiang Xia Acquisition of data: Qi-Chao Chen Analysis and interpretation of the data: Hong-Tao Zong Statistical analysis: Qi-Chao Chen, Qiang Xia Obtaining financing: Lian-Sheng Zhang Writing of the manuscript: Lian-Sheng Zhang, Qiang Xia Critical revision of the manuscript for intellectual content: Lian-Sheng Zhang, Hong-Tao Zong, Qiang Xia All authors read and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
The study was reviewed and approved by the Medical Ethics Committee of Wuxi Ninth Hospital (No. KT2022013), and the animal experiments were followed strictly by the approved guidelines.
Consent to participate
Urine samples were obtained with the informed consent of the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, LS., Chen, QC., Zong, HT. et al. Exosome miRNA-203 promotes M1 macrophage polarization and inhibits prostate cancer tumor progression. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04854-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04854-5