Abstract
The role of carbon monoxide (CO) has evolved albeit controversial disputes on its toxicity. This biological gasotransmitter participates in the endogenous regulation of neurotransmitters and neuropeptides released in the nervous system. Exogenous CO gas inhalation at a lower concentration has been the subject of investigations, which have revealed its biological homeostatic mechanisms and protective effects against many pathological conditions. This therapeutic procedure of CO is, however, limited due to its immediate release, which favours haemoglobin at a high affinity with the subsequent generation of toxic carboxyhaemoglobin in tissues. In order to address this problem, carbon monoxide releasing molecule-2 (CORM-2) or also known as tricarbonyldichlororuthenium II dimer is developed to liberate a controlled amount of CO in the biological systems. In this review, we examine several potential mechanisms exerted by this therapeutic compound to produce the anti-nociceptive effect that has been demonstrated in previous studies. This review could shed light on the role of CORM-2 to reduce pain, especially in cases of chronic and neuropathic pain.

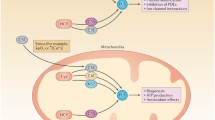
(Adapted from Southam et al. [91])

Similar content being viewed by others
Data availability
This is a review manuscript. Therefore, it does not contain original data to be available.
References
Douglas CG, Haldane JS, Haldane JBS (1912) The laws of combination of haemoglobin with carbon monoxide and oxygen. J Physiol 44:275–304. https://doi.org/10.1113/jphysiol.1912.sp001517
Haldane JBS (1927) Carbon monoxide as a tissue poison. Biochem J 21:1068–1075. https://doi.org/10.1042/bj0211068
Retamal MA (2016) Carbon monoxide modulates connexin function through a lipid peroxidation-dependent process: a hypothesis. Front Physiol. https://doi.org/10.3389/fphys.2016.00259
Coburn RF (1970) Biological effects of carbon monoxide. New York Academy of Sciences.
Poss KD, Tonegawa S (1997) Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci 94:10925–10930. https://doi.org/10.1073/PNAS.94.20.10925
Wu L, Wang R (2005) Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57:585–630. https://doi.org/10.1124/pr.57.4.3
Berrino E, Milazzo L, Micheli L, Vullo D, Angeli A, Bozdag M, Nocentini A, Menicatti M, Bartolucci G, Di Cesare ML, Ghelardini C, Supuran CT, Carta F (2019) Synthesis and evaluation of carbonic anhydrase inhibitors with carbon monoxide releasing properties for the management of rheumatoid arthritis. J Med Chem 62:7233–7249. https://doi.org/10.1021/acs.jmedchem.9b00845
Kealey GP (2009) Carbon monoxide toxicity. J Burn Care Res 30:146–147. https://doi.org/10.1097/BCR.0B013E3181923B81
Faizan M, Muhammad N, Niazi KUK, Hu Y, Wang Y, Wu Y, Sun H, Liu R, Dong W, Zhang W, Gao Z (2019) CO-releasing materials: an emphasis on therapeutic implications, as release and subsequent cytotoxicity are the part of therapy. Materials 12:1643. https://doi.org/10.3390/MA12101643
Ismailova A, Kuter D, Bohle DS, Butler IS (2018) An overview of the potential therapeutic applications of CO-releasing molecules. Bioinorg Chem Appl 2018:8547364. https://doi.org/10.1155/2018/8547364
Deshane J, Wright M, Agarwal A (2005) Heme oxygenase-1 expression in disease states. Acta Biochim Pol 52:273–284. https://doi.org/10.18388/ABP.2005_3440
Motterlini R, Otterbein LE (2010) The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 9:728–743. https://doi.org/10.1038/nrd3228
Foresti R, Bani-Hani MG, Motterlini R (2008) Use of carbon monoxide as a therapeutic agent: promises and challenges. Intensive Care Med 34:649–658. https://doi.org/10.1007/s00134-008-1011-1
Lee GY, Zeb A, Kim EH, Suh B, Shin YJ, Kim D, Kim KW, Choe YH, Choi HI, Lee CH, Qureshi OS, Han IB, Chang SY, Bae ON, Kim JK (2020) CORM-2-entrapped ultradeformable liposomes ameliorate acute skin inflammation in an ear edema model via effective CO delivery. Acta Pharm Sin B 10:2362–2373. https://doi.org/10.1016/j.apsb.2020.05.010
Romão CC, Blättler WA, Seixas JD, Bernardes GJL (2012) Developing drug molecules for therapy with carbon monoxide. Chem Soc Rev 41:3571–3583. https://doi.org/10.1039/c2cs15317c
Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ (2002) Carbon monoxide-releasing molecules. Circ Res. https://doi.org/10.1161/HH0202.104530
Ferrándiz ML, Maicas N, Garcia-Arnandis I, Terencio MC, Motterlini R, Devesa I, Joosten LAB, Van Den Berg WB, Alcaraz MJ (2008) Treatment with a CO-releasing molecule (CORM-3) reduces joint inflammation and erosion in murine collagen-induced arthritis. Ann Rheum Dis 67:1211–1217. https://doi.org/10.1136/ard.2007.082412
Juszczak M, Kluska M, Wysokiński D, Woźniak K (2020) DNA damage and antioxidant properties of CORM-2 in normal and cancer cells. Sci Reports 101:1–12. https://doi.org/10.1038/s41598-020-68948-6
Zamani M, Ale YA, Fakhrzadeh H, Kiavar M, Raoufzadeh S, Mahmoudi E (2010) Heme oxigenase 2 gene polymorphisms as genetic risk factor in atherosclerosis in Iranian patients. Iran Red Crescent Med J 2010:559–563. https://www.sid.ir/journal/1602/en
Motterlini R, Haas B, Foresti R (2012) Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs). Med Gas Res 2:28. https://doi.org/10.1186/2045-9912-2-28
Jurga AM, Piotrowska A, Makuch W, Przewlocka B, Mika J (2017) Blockade of P2X4 receptors inhibits neuropathic pain-related behavior by preventing MMP-9 activation and consequently, pronociceptive interleukin release in a rat model. Front Pharmacol 8:1–18. https://doi.org/10.3389/fphar.2017.00048
Méndez-Lara KA, Santos D, Farré N, Ruiz-Nogales S, Leánez S, Sánchez-Quesada JL, Zapico E, Lerma E, Escola-Gil JC, Blanco-Vaca F, Martón-Campos JM, Julve J, Pol O (2018) Administration of CORM-2 inhibits diabetic neuropathy but does not reduce dyslipidemia in diabetic mice. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0204841
Soni H, Pandya G, Patel P, Acharya A, Jain M, Mehta AA (2011) Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: role of oxidative stress and apoptosis. Toxicol Appl Pharmacol 253:70–80. https://doi.org/10.1016/J.TAAP.2011.03.013
Babu D, Leclercq G, Motterlini R, Lefebvre RA (2017) Differential effects of CORM-2 and CORM-401 in murine intestinal epithelial MODE-K cells under oxidative stress. Front Pharmacol 8:31. https://doi.org/10.3389/FPHAR.2017.00031/BIBTEX
Wang J, Zhang D, Fu X, Yu L, Lu Z, Gao Y, Liu X, Man J, Li S, Li N, Chen X, Hong M, Yang Q, Wang J (2018) Carbon monoxide-releasing molecule-3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood-brain barrier disruption. J Neuroinflammation 15:1–13. https://doi.org/10.1186/S12974-018-1226-1/FIGURES/6
Chu LM, Shaefi S, Byrne JD, Alves de Souza RW, Otterbein LE (2021) Carbon monoxide and a change of heart. Redox Biol. https://doi.org/10.1016/J.REDOX.2021.102183
Yan BZ, Yang BS, Li H, Zhang YF, Pei FH, Zhu AC, Wang XR, Liu BR (2016) The therapeutic effect of CORM-3 on acute liver failure induced by lipopolysaccharide/D-galactosamine in mice. Hepatobiliary Pancreat Dis Int 15:73–80. https://doi.org/10.1016/S1499-3872(15)60044-3
Zhang DD, Liang YF, Qi J, Kang KB, Yu XJ, Gao HL, Liu KL, Chen YM, Shi XL, Xin GR, Fu LY, Kang YM, Cui W (2019) Carbon monoxide attenuates high salt-induced hypertension while reducing pro-inflammatory cytokines and oxidative stress in the paraventricular nucleus. Cardiovasc Toxicol 19:451–464. https://doi.org/10.1007/S12012-019-09517-W
Wang P, Yao L, Zhou L-L, Liu Y-S, Chen M-D, Wu H-D, Chang R-M, Li Y, Zhou M-G, Fang X-S, Yu T, Jiang L-Y, Huang Z-T (2016) Carbon monoxide improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. Int J Biol Sci 12:1000–1009. https://doi.org/10.7150/ijbs.13222
Motterlini R, Clark E, Foresti R, Sarathchandra P, Mann BE, Green CJ (2002) Carbon monoxide-releasing molecules characterization of Biochemical and vascular activities. Circ Res 90:e17–e24. https://doi.org/10.1161/hh0202.104530
Liu Y, Wang X, Xu X, Qin W, Sun B (2019) Carbon monoxide releasing molecule-2 (CORM-2)-liberated CO ameliorates acute pancreatitis. Mol Med Rep 19:5142–5152. https://doi.org/10.3892/MMR.2019.10173/HTML
Wilkinson WJ, Kemp PJ (2011) The carbon monoxide donor, CORM-2, is an antagonist of ATP-gated, human P2X4 receptors. Purinergic Signal 7:57–64. https://doi.org/10.1007/s11302-010-9213-8
Zobi F (2013) CO and CO-releasing molecules in medicinal chemistry. Future Med Chem 5:175–188. https://doi.org/10.4155/fmc.12.196
Simpson PV, Schatzschneider U (2016) Small signaling molecules and CO-releasing molecules (CORMs) for the modulation of the cellular redox metabolism. In: Batinić-Haberle I, Rebouças J, Spasojević I (eds) Redox-active therapeutics Oxidative stress in applied basic research and clinical practice. Springer, Cham, pp 311–334. https://doi.org/10.1007/978-3-319-30705-3_13
Joshi HP, Kim SB, Kim S, Kumar H, Jo M-J, Choi H, Kim J, Kyung JW, Sohn S, Kim K-T, Kim J-K, Han I-B (2019) Nanocarrier-mediated delivery of CORM-2 enhances anti-allodynic and anti-hyperalgesic effects of CORM-2. Mol Neurobiol 56:5539–5554. https://doi.org/10.1007/s12035-019-1468-7
Qureshi OS, Zeb A, Akram M, Kim MS, Kang JH, Kim HS, Majid A, Han I, Chang SY, Bae ON, Kim JK (2016) Enhanced acute anti-inflammatory effects of CORM-2-loaded nanoparticles via sustained carbon monoxide delivery. Eur J Pharm Biopharm 108:187–195. https://doi.org/10.1016/j.ejpb.2016.09.008
Yin H, Fang J, Liao L, Nakamura H, Maeda H (2014) Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J Contr Release 187:14–21. https://doi.org/10.1016/j.jconrel.2014.05.018
Natarajan JV, Nugraha C, Ng XW, Venkatraman S (2014) Sustained-release from nanocarriers: a review. J Contr Release 193:122–138. https://doi.org/10.1016/J.JCONREL.2014.05.029
Dong DL, Chen C, Huang W, Chen Y, Zhang XL, Li Z, Li Y, Yang BF (2008) Tricarbonyldichlororuthenium (II) dimer (CORM2) activates non-selective cation current in human endothelial cells independently of carbon monoxide releasing. Eur J Pharmacol 590:99–104. https://doi.org/10.1016/J.EJPHAR.2008.05.042
Wilkinson WJ, Kemp PJ (2011) Carbon monoxide: an emerging regulator of ion channels. J Physiol 589:3055–3062. https://doi.org/10.1113/jphysiol.2011.206706
Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C (2008) Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem 283:24412–24419. https://doi.org/10.1074/jbc.M803037200
Joshi HP, Kumar H, Choi UY, Lim YC, Choi H, Kim J, Kyung JW, Sohn S, Kim KT, Kim JK, Han IB (2020) CORM-2-solid lipid nanoparticles maintain integrity of blood-spinal cord barrier after spinal cord injury in rats. Mol Neurobiol 57:2671–2689. https://doi.org/10.1007/s12035-020-01914-5
Antonioli L, Blandizzi C, Pacher P, Haskó G (2019) The purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases. Pharmacol Rev 71:345–382. https://doi.org/10.1124/PR.117.014878
Kong Q, Quan Y, Tian G, Zhou J, Liu X (2021) Purinergic P2 receptors: novel mediators of mechanotransduction. Front Pharmacol 12:1099. https://doi.org/10.3389/FPHAR.2021.671809/BIBTEX
Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K (2005) Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol 481:377–390. https://doi.org/10.1002/cne.20393
Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P (2014) Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature Neurosci 18:145–153. https://doi.org/10.1038/nn.3881
Di Virgilio F, Sarti AC (2018) Microglia P2X4 receptors as pharmacological targets for demyelinating diseases. EMBO Mol Med. https://doi.org/10.15252/emmm.201809369
Kohno K, Tsuda M (2021) Role of microglia and P2X4 receptors in chronic pain. Pain Rep. https://doi.org/10.1097/pr9.0000000000000864
Inoue K (2017) Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc Japan Acad Ser B 93:174–182. https://doi.org/10.2183/PJAB.93.011
Trang T, Beggs S, Wan X, Salter MW (2009) P2S4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29:3518–3528. https://doi.org/10.1523/JNEUROSCI.5714-08.2009
Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28:11263–11268. https://doi.org/10.1523/JNEUROSCI.2308-08.2008
Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C (2008) Carbon Monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem 283:24412–24419. https://doi.org/10.1074/JBC.M803037200
Wang H, Sun X (2017) Carbon monoxide-releasing molecule-2 Inhibits connexin 43-hemichannel activity in spinal cord astrocytes to attenuate neuropathic pain. J Mol Neurosci 63:58–69. https://doi.org/10.1007/s12031-017-0957-2
Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M (2012) Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60:1660–1670. https://doi.org/10.1002/GLIA.22384
Srisook K, Han SS, Choi HS, Li MH, Ueda H, Kim C, Cha YN (2006) CO from enhanced HO activity or from CORM-2 inhibits both O2− and NO production and downregulates HO-1 expression in LPS-stimulated macrophages. Biochem Pharmacol 71:307–318. https://doi.org/10.1016/J.BCP.2005.10.042
Hervera A, Leánez S, Negrete R, Motterlini R, Pol O (2012) Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0043693
Jurga AM, Piotrowska A, Starnowska J, Rojewska E, Makuch W, Mika J (2016) Treatment with a carbon monoxide-releasing molecule (CORM-2) inhibits neuropathic pain and enhances opioid effectiveness in rats. Pharmacol Rep 68:206–213. https://doi.org/10.1016/j.pharep.2015.08.016
Adach W, Olas B (2017) The role of CORM-2 as a modulator of oxidative stress and hemostatic parameters of human plasma in vitro. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0184787
Tsai MH, Lee CW, Hsu LF, Li SY, Chiang YC, Lee MH, Chen CH, Liang HF, How JM, Chang PJ, Wu CM, Lee IT (2017) CO-releasing molecules CORM2 attenuates angiotensin II-induced human aortic smooth muscle cell migration through inhibition of ROS/IL-6 generation and matrix metalloproteinases-9 expression. Redox Biol 12:377–388. https://doi.org/10.1016/J.REDOX.2017.02.019
Rochette L, Cottin Y, Zeller M, Vergely C (2013) Carbon monoxide: Mechanisms of action and potential clinical implications. Pharmacol Ther 137:133–152. https://doi.org/10.1016/J.PHARMTHERA.2012.09.007
Castany S, Carcolé M, Leánez S, Pol O (2016) The role of carbon monoxide on the anti-nociceptive effects and expression of cannabinoid 2 receptors during painful diabetic neuropathy in mice. Psychopharmacology 233:2209–2219. https://doi.org/10.1007/s00213-016-4271-4
Hervera A, Gou G, Leánez S, Pol O (2013) Effects of treatment with a carbon monoxide-releasing molecule and a heme oxygenase 1 inducer in the antinociceptive effects of morphine in different models of acute and chronic pain in mice. Psychopharmacology 228:463–477. https://doi.org/10.1007/s00213-013-3053-5
Bailey CP, Connor M (2005) Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68. https://doi.org/10.1016/J.COPH.2004.08.012
Schembri E (2018) Are opioids effective in relieving neuropathic pain? SN Compr Clin Med 1:30–46. https://doi.org/10.1007/S42399-018-0009-4
Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: novel counter-regulators of opioid analgesia. Trends Neurosci 28:661–669. Glia: novel counter-regulators of opioid analgesia
Mika J, Osikowicz M, Makuch W, Przewlocka B (2007) Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol 560:142–149. https://doi.org/10.1016/J.EJPHAR.2007.01.013
Machelska H, Celik M (2020) Opioid receptors in immune and glial cells—implications for pain control. Front Immunol 11:1–13. https://doi.org/10.3389/fimmu.2020.00300
Ehrlich AT, Kieffer BL, Darcq E (2019) Current strategies toward safer mu opioid receptor drugs for pain management. Expert Opin Ther Targets 23:315–326. https://doi.org/10.1080/14728222.2019.1586882
Gavériaux-Ruff C, Kieffer BL (2011) Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol 22:405. https://doi.org/10.1097/FBP.0B013E32834A1F2C
Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM, Cahill CM (2019) Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci 39:4162–4178. https://doi.org/10.1523/JNEUROSCI.0274-19.2019
Machelska H, Celik M (2018) Advances in achieving opioid analgesia without side effects. Front Pharmacol 9:1388. https://doi.org/10.3389/FPHAR.2018.01388/BIBTEX
Cazuza RA, Arantes ALF, Pol O, Leite-Panissi CRA (2021) HO-CO pathway activation may be associated with hippocampal μ and δ opioid receptors in inhibiting inflammatory pain aversiveness and nociception in WT but not NOS2-KO mice. Brain Res Bull 169:8–17. https://doi.org/10.1016/j.brainresbull.2021.01.002
Vareniuk I, Pacher P, Pavlov IA, Drel VR, Obrosova IG (2009) Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int J Mol Med 23:571–580. https://doi.org/10.3892/IJMM_00000166/HTML
Negrete R, Hervera A, Leánez S, Pol O (2014) Treatment with a carbon monoxide-releasing molecule inhibits chronic inflammatory pain in mice: Nitric oxide contribution. Psychopharmacology 231:853–861. https://doi.org/10.1007/s00213-013-3302-7
Boettger MK, Üceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C (2007) Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain 11:810–818. https://doi.org/10.1016/J.EJPAIN.2006.12.008
Leánez S, Hervera A, Pol O (2009) Peripheral antinociceptive effects of µ- and δ-opioid receptor agonists in NOS2 and NOS1 knockout mice during chronic inflammatory pain. Eur J Pharmacol 602:41–49. https://doi.org/10.1016/J.EJPHAR.2008.11.019
Gou G, Leánez S, Pol O (2014) The role of gaseous neurotransmitters in the antinociceptive effects of morphine during acute thermal pain. Eur J Pharmacol 737:41–46. https://doi.org/10.1016/j.ejphar.2014.05.004
Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR (2018) Microglia in Pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 100:1292–1311. https://doi.org/10.1016/j.neuron.2018.11.009
Steiner AA, Branco LGS, Cunha FQ, Ferreira SH (2001) Role of the heme oxygenase/carbon monoxide pathway in mechanical nociceptor hypersensitivity. British J Pharmacol 132:1673–1682. https://doi.org/10.1038/sj.bjp.0704014
Nascimento CGO, Branco GS (2006) Role of the peripheral heme oxygenase-carbon monoxide pathway on the nociceptive response of rats to the formalin test: evidence for a cGMP signaling pathway. Eur J Pharmacol 556:55–61. https://doi.org/10.1016/j.ejphar.2006.10.009
de Ávila MAP, Giusti-Paiva A, Nascimento CGO (2014) The peripheral antinociceptive effect induced by the heme oxygenase/carbon monoxide pathway is associated with ATP-sensitive K+ channels. Eur J Pharmacol 726:41–48. https://doi.org/10.1016/J.EJPHAR.2014.01.012
Godai K, Kanmura Y (2018) Heme oxygenase-1 inducer and carbon monoxide-releasing molecule enhance the effects of gabapentinoids by modulating glial activation during neuropathic pain in mice. Pain Rep. https://doi.org/10.1097/PR9.0000000000000677
Riego G, Redondo A, Leánez S, Pol O (2018) Mechanism implicated in the anti-allodynic and anti-hyperalgesic effects induced by the activation of heme oxygenas 1/carbon monoxide signaling pathway in the central nervous system of mice with neuropathic pain. Biochem Pharmacol 148:52–63. https://doi.org/10.1016/j.bcp.2017.12.007
Woo J-I, Kil S-H, Oh S, Lee Y-J, Park R, Lim DJ, Moon SK (2015) IL-10/HMOX1 signaling modulates cochlear inflammation via negative regulation of MCP-1/CCL2 expression in cochlear fibrocytes. J Immunol 194:3953–3961. https://doi.org/10.4049/JIMMUNOL.1402751
Riquelme SA, Bueno SM, Kalergis AM (2015) Carbon monoxide down-modulates Toll-like receptor 4/MD2 expression on innate immune cells and reduces endotoxic shock susceptibility. Immunology 144:321–332. https://doi.org/10.1111/IMM.12375
Lacagnina MJ, Watkins LR, Grace PM (2018) Toll-like receptors and their role in persistent pain. Pharmacol Ther 184:145–158. https://doi.org/10.1016/J.PHARMTHERA.2017.10.006
Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W (2002) Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res 55:396–405. https://doi.org/10.1016/S0008-6363(02)00410-8/2/55-2-396-FIG7.GIF
Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899. https://doi.org/10.1126/SCIENCE.1079368/SUPPL_FILE/NISOLI.SOM.PDF
Xie Z, Han P, Cui Z, Wang B, Zhong Z, Sun Y, Yang G, Sun Q, Bian L (2016) Pretreatment of mouse neural stem cells with carbon monoxide-releasing molecule-2 interferes with NF-κB p65 signaling and suppresses iron overload-induced apoptosis. Cell Mol Neurobiol 36:1343–1351. https://doi.org/10.1007/s10571-016-0333-8
Faizan M, Niazi KUK, Muhammad N, Hu Y, Wang Y, Lin D, Liu Y, Zhang W, Gao Z (2019) The intercalation of CORM-2 with pharmaceutical clay montmorillonite (MMT) aids for therapeutic carbon monoxide release. Int J Mol Sci 20:3453. https://doi.org/10.3390/IJMS20143453
Southam HM, Williamson MP, Chapman JA, Lyon RL, Trevitt CR, Henderson PJF, Poole RK (2021) ‘Carbon-monoxide-releasing molecule-2 (CORM-2)’ is a misnomer: ruthenium toxicity, not CO release, accounts for its antimicrobial effects. Antioxidants 10:915. https://doi.org/10.3390/antiox10060915
Bijjem KRV, Padi SSV, Pl S (2013) Pharmacological activation of heme oxygenase (HO)-1/carbon monoxide pathway prevents the development of peripheral neuropathic pain in Wistar rats. Naunyn-Schmiedeb Arch Pharmacol 386:79–90. https://doi.org/10.1007/s00210-012-0816-1
Hervera A, Leánez S, Motterlini R, Pol O (2016) Treatment with carbon monoxide-releasing molecules and an HO-1 inducer enhances the effects and expression of μ-opioid receptors during neuropathic pain. Anesthesiology 118:1180–1197. https://doi.org/10.1097/ALN.0b013e318286d085
Liu X, Zhang Z, Cheng Z, Zhang J, Xu S, Liu H, Jia H, Jin Y (2016) Spinal Heme oxygenase-1 (HO-1) exerts antinociceptive effects against neuropathic pain in a mouse model of L5 spinal nerve ligation. Pain Med 17:220–229. https://doi.org/10.1111/pme.12906
Castany S, Carcolé M, Leánez S, Pol O (2016) The antinociceptive effects of a δ-opioid receptor agonist in mice with painful diabetic neuropathy: Involvement of heme oxygenase 1. Neurosci Lett 614:49–54. https://doi.org/10.1016/j.neulet.2015.12.059
Moreno P, Cazuza RA, Gomes-Mendes J, Díaz AF, Polo S, Leánez S, Leite-Panissi CRA, Pol O (2019) The effects of cobalt protoporphyrin IX and tricarbonyldichlororuthenium (II) dimer treatments and its interaction with nitric oxide in the locus coeruleus of mice with peripheral inflammation. Int J Mol Sci 20:2211. https://doi.org/10.3390/ijms20092211
Acknowledgements
We would like to thank Ministry of Higher Education Malaysia for providing research grant (Fundamental Research Grant Scheme Project Code: FRGS/1/2019/SKK08/USM/03/13) in order to further exploring the therapeutic effects of CORM-2 in chronic pain.
Funding
This work was supported by Ministry of Higher Education Malaysia [Fundamental Research Grant Scheme Project Code: FRGS/1/2019/SKK08/USM/03/13] received by Ismail CAN.
Author information
Authors and Affiliations
Contributions
Material preparation and data collection were performed by Khir NAM and Noh ASM. The manuscript draft was written by Khir NAM and Ismail CAN commented on the previous manuscript drafts. Long I and Zakaria R has reviewed and proofread the prepared manuscript, All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a review manuscript. Universiti Sains Malaysia (USM) Institutional Animal Care and Use Committee (USM IACUC) and USM Human Research Ethics Committee have confirmed that no ethical approval is required.
Informed consent
Informed consent is not applicable for the prepared review manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khir, N.A.M., Noh, A.S.M., Long, I. et al. Recent progress on anti-nociceptive effects of carbon monoxide releasing molecule-2 (CORM-2). Mol Cell Biochem 479, 539–552 (2024). https://doi.org/10.1007/s11010-023-04749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04749-5