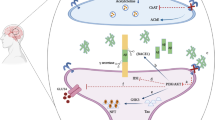
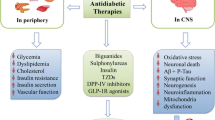
Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder affecting a major class of silver citizens. The disorder shares a mutual relationship on account of its cellular and molecular pathophysiology with type-II diabetes mellitus (DM). Chronic DM increases the risk for AD. Emerging evidence recommended that resistance in insulin production develops cognitive dysfunction, which generally leads to AD. Repurposing of antidiabetic drugs can be effective in preventing and treatment of the neurodegenerative disorder. Limitations of antidiabetic drugs restrict the repurposing of the drugs for other disorders. Therefore, nanotechnological intervention plays a significant role in the treatment of neurological disorders. In this review, we discuss the common cellular and molecular pathophysiologies between AD and type-II DM, the relevance of in vivo models of type II DM in the study of AD, and the repurposing of antidiabetic drugs and the nanodelivery systems of antidiabetic drugs against AD.
Graphical abstract



Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Baranowska-Wójcik E, Szwajgier D (2020) Alzheimer’s disease: review of current nanotechnological therapeutic strategies. Expert Rev Neurother 20:271–279. https://doi.org/10.1080/14737175.2020.1719069
WHO (2020) Dementia fact sheet. World Health Organization, Geneva
Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K et al (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38:1205–1235. https://doi.org/10.1038/aps.2017.28
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:1–8. https://doi.org/10.1371/journal.pone.0032792
Savelieff MG, Nam G, Kang J, Lee HJ, Lee M, Lim MH (2018) Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis in the last decade. Chem Rev 119:1221–1322. https://doi.org/10.1021/acs.chemrev.8b00138
Guzman-Martinez L, Calfío C, Farias GA, Vilches C, Prieto R, Maccioni RB (2021) New frontiers in the prevention, diagnosis, and treatment of Alzheimer’s disease. J Alzheimer’s Dis 82:S51-63. https://doi.org/10.3233/JAD-201059
Abyadeh M, Gupta V, Gupta V, Chitranshi N, Wu Y, Amirkhani A et al (2021) Comparative analysis of aducanumab, zagotenemab and pioglitazone as targeted treatment strategies for Alzheimer’s disease. Aging Dis 12:1964. https://doi.org/10.14336/AD.2021.0719
Baruah P, Das A, Paul D, Chakrabarty S, Aguan K, Mitra S (2021) Sulfonylurea class of antidiabetic drugs inhibit acetylcholinesterase activity: unexplored auxiliary pharmacological benefit toward Alzheimer’s disease. ACS Pharmacol Transl Sci 4:193–205. https://doi.org/10.1021/acsptsci.0c00168
Muñoz-Jiménez M, Zaarkti A, García-Arnés JA, García-Casares N (2020) Antidiabetic drugs in Alzheimer’s disease and mild cognitive impairment: a systematic review. Dement Geriatr Cogn Dis 4911111:423–434. https://doi.org/10.1248/bpb.b14-00819
Santiago JA, Potashkin JA (2021) The impact of disease comorbidities in Alzheimer’s disease. Front Aging Neurosci 13:38. https://doi.org/10.3389/fnagi.2021.631770
Padhi S, Nayak AK, Behera A (2020) Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother 131:110708. https://doi.org/10.1016/j.biopha.2020.110708
Verma N, Despa F (2019) Contributing factors to diabetic brain injury and cognitive decline. Diabetes Metab J 43:560. https://doi.org/10.4093/dmj.2019.0153
Padhi S, Dash M, Behera A (2021) Nanophytochemicals for the treatment of type II diabetes mellitus: a review. Environ Chem Lett 19:4349–4373. https://doi.org/10.1007/s10311-021-01283-y
Chawla A, Chawla R, Jaggi S (2016) Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab 20:546. https://doi.org/10.4103/2230-8210.183480
Maranta F, Cianfanelli L, Cianflone D (2020) Glycaemic control and vascular complications in diabetes mellitus type 2. Adv Exp Med Biol 1307:129–152. https://doi.org/10.1007/5584_2020_514
Bosco D, Plastino M, Cristiano D, Colica C, Ermio C, De Bartolo M et al (2012) Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci 315:39–43. https://doi.org/10.1016/j.jns.2011.12.008
De la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes—evidence reviewed. J Diabetes Sci Technol 2:1101–1113. https://doi.org/10.1177/193229680800200619
Klimova B, Kuca K, Maresova P (2018) Global view on Alzheimer’s Disease and Diabetes Mellitus: threats, risks and treatment Alzheimer’s Disease and Diabetes Mellitus. Curr Alzheimer Res 15:1277–1282. https://doi.org/10.2174/1567205015666180925110222
An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O et al (2018) Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s Dement 14:318–329. https://doi.org/10.1016/j.jalz.2017.09.011
Suzuki T, Nakaya T (2008) Regulation of amyloid β-protein precursor by phosphorylation and protein interactions. J Biol Chem 283:29633–29637. https://doi.org/10.1074/jbc.R800003200
Liao YH, Chang YJ, Yoshiike Y, Chang YC, Chen YR (2012) Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-β fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small 8:3631–3639. https://doi.org/10.1002/smll.201201068
Kumar K, Kumar A, Keegan RM, Deshmukh R (2018) Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed Pharmacother 98:297–307. https://doi.org/10.1016/j.biopha.2017.12.053
Busquets MA, Sabaté R, Estelrich J (2014) Potential applications of magnetic particles to detect and treat Alzheimer’s disease. Nanoscale Res Lett 9:1–10. https://doi.org/10.1186/1556-276X-9-538
Chakravarthy M, Chen S, Dodd PR, Veedu RN (2017) Nucleic acid-based theranostics for tackling Alzheimer’s disease. Theranostics 7:3933. https://doi.org/10.7150/thno.21529
Peric A, Annaert W (2015) Early etiology of Alzheimer’s disease: tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol 129:363–381. https://doi.org/10.1007/s00401-014-1379-7
Barret KE, Boitano S, Barman SM (2012) Ganong’s review of medical physiology. McGraw-Hill Medical, New York
Kumar V, Sami N, Kashav T, Islam A, Ahmad F, Hassan MI (2016) Protein aggregation and neurodegenerative diseases: from theory to therapy. Eur J Med Chem 124:1105–1120. https://doi.org/10.1016/j.ejmech.2016.07.054
Xu W, Xu Q, Cheng H, Tan X (2017) The efficacy and pharmacological mechanism of Zn 7 MT3 to protect against Alzheimer’s disease. Sci Rep 7:1–15. https://doi.org/10.1038/s41598-017-12800-x
Abeysinghe AADT, Deshapriya RDUS, Udawatte C (2020) Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci 256:117996. https://doi.org/10.1016/j.lfs.2020.117996
González A, Singh SK, Churruca M, Maccioni RB (2022) Alzheimer’s disease and tau self-assembly: in the search of the missing link. Int J Mol Sci 23:4192. https://doi.org/10.3390/ijms23084192
DeFina PA, Moser RS, Glenn M, Lichtenstein JD, Fellus J (2013) Alzheimer’s disease clinical and research update for health care practitioners. J Aging Res. https://doi.org/10.1155/2013/207178
Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K (2013) Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimer’s Dis 33:S123–S139. https://doi.org/10.3233/JAD-2012-129031
Komane PP, Choonara YE, du Toit LC, Kumar P, Kondiah PP, Modi G et al (2016) Diagnosis and treatment of neurological and ischemic disorders employing carbon nanotube technology. J Nanomater. https://doi.org/10.1155/2016/9417874
Sahu PK, Tiwari P, Prusty SK, Subudhi BB (2018) Past and present drug development for Alzheimer’s disease. In: Frontiers in clinical drug research—Alzheimer disorders. Bentham Science Publishers. eISBN 978-1-68108-560-569, ISBN 978-1-681087:561-566
Rammohan A, Reddy J, Sravya G, Rao C, Zyryanov G (2020) Chalcone synthesis, properties and medicinal applications: a review. Environ Chem Lett 18:433–458. https://doi.org/10.1007/s10311-019-00959-w
Unuofin JO, Lebelo SL (2020) Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: an updated review. Oxid Med Cell Longev 2020:1356893
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB et al (2020) Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 21:6275. https://doi.org/10.3390/ijms21176275
Yaribeygi H, Atkin SL, Sahebkar A (2019) A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J Cell Physiol 234:1300–1312. https://doi.org/10.1002/jcp.27164
Ohiagu FO, Chikezie PC, Chikezie CM (2021) Pathophysiology of diabetes mellitus complications: metabolic events and control. Biomed Res Ther 8:4243–4257
Sharavana G, Joseph GS, Baskaran V (2017) Lutein attenuates oxidative stress markers and ameliorates glucose homeostasis through polyol pathway in heart and kidney of STZ-induced hyperglycemic rat model. Eur J Nutr 56:2475–2485. https://doi.org/10.1007/s00394-016-1283-0
Naidu PB, Uddandrao VVS, Naik RR, Pothani S, Munipally PK, Meriga B et al (2016) Effects of S-allylcysteine on biomarkers of the polyol pathway in rats with type 2 diabetes. Can J Diabetes 40:442–448. https://doi.org/10.1016/j.jcjd.2016.03.006
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H et al (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Investig 122:1339–1353. https://doi.org/10.1172/JCI57256
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A et al (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Investig 122:1316–1338. https://doi.org/10.1172/JCI59903
Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 1863:1078–1089. https://doi.org/10.1016/j.bbadis.2016.08.018
Pang Y, Lin S, Wright C, Shen J, Carter K, Bhatt A et al (2016) Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience 318:157–165. https://doi.org/10.1016/j.neuroscience.2016.01.020
Vieira MN, Lima-Filho RA (2018) De Felice FG (2018) Connecting Alzheimer’s disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology 136:160–171
Bharadwaj P, Wijesekara N, Liyanapathirana M, Newsholme P, Ittner L, Fraser P et al (2017) The link between type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J Alzheimer’s Dis 59:421–432. https://doi.org/10.3233/JAD-161192
Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL (2003) Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci 23:7084–7092
Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E, Arndt S (2004) Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101:3100–3105. https://doi.org/10.1073/pnas.0308724101
Zhang Y, Huang NQ, Yan F, Jin H, Zhou SY, Shi JS et al (2018) Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav Brain Res 339:57–65
Takano K, Koarashi K, Kawabe K, Itakura M, Nakajima H, Moriyama M et al (2018) Insulin expression in cultured astrocytes and the decrease by amyloid β. Neurochem Int 119:171–177. https://doi.org/10.1016/j.neuint.2017.10.017
Kamynina AV, Esteras N, Koroev DO, Bobkova NV, Balasanyants SM, Simonyan RA et al (2018) Synthetic fragments of receptor for advanced glycation end products bind beta-amyloid 1–40 and protect primary brain cells from beta-amyloid toxicity. Front Neurosci 12:681. https://doi.org/10.3389/fnins.2018.00681
Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5:194–222. https://doi.org/10.3390/biom5010194
Singh VP, Bali A, Singh N, Jaggi AS (2014) Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 18:1–4. https://doi.org/10.4196/kjpp.2014.18.1.1
Baig MH, Ahmad K, Rabbani G, Choi I (2018) Use of peptides for the management of Alzheimer’s disease: diagnosis and inhibition. Front Aging Neurosci 10:21. https://doi.org/10.3389/fnagi.2018.00021
Kong Y, Wang F, Wang J, Liu C, Zhou Y, Xu Z et al (2020) Pathological mechanisms linking diabetes mellitus and Alzheimer’s disease: the receptor for advanced glycation end products (RAGE). Front Aging Neurosci 12:217. https://doi.org/10.3389/fnagi.2020.00217
Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X et al (2015) Aβ1–42 oligomer-induced leakage in an in vitro blood–brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem 134:382–393. https://doi.org/10.1111/jnc.13122
Fang F, Yu Q, Arancio O, Chen D, Gore SS, Yan SS et al (2018) RAGE mediates Aβ accumulation in a mouse model of Alzheimer’s disease via modulation of β- and γ-secretase activity. Hum Mol Genet 27:1002–1014. https://doi.org/10.1093/hmg/ddy017
Wu B, Wang Y, Shi C, Chen Y, Yu L, Li J et al (2019) Ribosylation-derived advanced glycation end products induce tau hyperphosphorylation through brain-derived neurotrophic factor reduction. J Alzheimer’s Dis 71:291–305. https://doi.org/10.3233/JAD-190158
Nam JH, Park KW, Park ES, Lee YB, Lee HG, Baik HH et al (2012) Interleukin-13/-4-induced oxidative stress contributes to death of hippocampal neurons in aβ1-42-treated hippocampus in vivo. Antioxid Redox Signal 16:1369–1383. https://doi.org/10.1089/ars.2011.4175
Cai Z, Liu N, Wang C, Qin B, Zhou Y, Xiao M et al (2016) Role of RAGE in Alzheimer’s disease. Cell Mol Neurobiol 36:483–495. https://doi.org/10.1007/s10571-015-0233-3
Huang Y, Mahley RW (2014) Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis 72:3–12. https://doi.org/10.1016/j.nbd.2014.08.025
Roses MDAD (1996) Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med 47:387–400. https://doi.org/10.1146/annurev.med.47.1.387
Ferrucci L, Guralnik JM, Pahor M, Harris T, Corti MC, Hyman BT, Wallace RB, Havlik RJ (1997) Apolipoprotein E ε2 and risk of stroke in the older population. J Stroke 28:2410–2416. https://doi.org/10.1161/01.STR.28.12.2410
Gonzalez-Dominguez R, Castellano-Escuder P, Lefèvre-Arbogast S, Low DY, Du Preez A, Ruigrok SR, Lee H, Helmer C, Pallàs M, Urpi-Sarda M, Sanchez-Pla A (2022) Apolipoprotein E and sex modulate fatty acid metabolism in a prospective observational study of cognitive decline. Alzheimer’s Res Ther 14:1–2. https://doi.org/10.1186/s13195-021-00948-8
Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G (2007) Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 56:66–78. https://doi.org/10.1016/j.neuron.2007.08.008
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10:333–344. https://doi.org/10.1038/nrn2620
Rajamani U (2014) Causes of neurodegeneration in diabetes: possible culprits and therapeutic targets. Brain Disord Ther 3:1–6. https://doi.org/10.4172/2168-975X.1000130
Tudorache IF, Trusca VG, Gafencu AV (2017) Apolipoprotein E—a multifunctional protein with implications in various pathologies as a result of its structural features. Comput Struct Biotechnol J 15:359–365. https://doi.org/10.1016/j.csbj.2017.05.003
Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35:669–675. https://doi.org/10.1016/j.tibs.2010.07.003
Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A (2017) Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther 23:5–22. https://doi.org/10.1111/cns.12655
Wijesekara N, Gonçalves RA, De Felice FG, Fraser PE (2018) Impaired peripheral glucose homeostasis and Alzheimer’s disease. Neuropharmacology 136:172–181. https://doi.org/10.1016/j.neuropharm.2017.11.027
Choi J, Ravipati A, Nimmagadda V, Schubert M, Castellani RJ, Russell JW (2014) Potential roles of PINK1 for increased PGC-1α-mediated mitochondrial fatty acid oxidation and their associations with Alzheimer disease and diabetes. Mitochondrion 18:41–48. https://doi.org/10.1016/j.mito.2014.09.005
Sun Y, Ma C, Sun H, Wang H, Peng W, Zhou Z et al (2020) Metabolism: a novel shared link between diabetes mellitus and Alzheimer’s disease. J Diabetes Res. https://doi.org/10.1155/2020/4981814
Nikooyeh B, Neyestani TR (2016) Oxidative stress, type 2 diabetes and vitamin D: past, present and future. Diabetes Metab Res Rev 32:260–267. https://doi.org/10.1002/dmrr.2718
Kowluru RA, Mishra M (2015) Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis 1852:2474–2483. https://doi.org/10.1016/j.bbadis.2015.08.001
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1842:1240–1247. https://doi.org/10.1016/j.bbadis.2013.10.015
Kalmijn S, Janssen JAMJL, Pols HAP, Lamberts SWJ, Breteler MMB (2000) A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab 85:4551–4555
Ferreira ST, Clarke JR, Bomfim TR, De Felice FG (2014) Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s Dement 10:S76–S83. https://doi.org/10.1016/j.jalz.2013.12.010
Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett-Connor E (2006) Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging 10:293
Decourt BK, Lahiri DN, Sabbagh M (2017) Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr Alzheimer’s Res 14:412–425. https://doi.org/10.2174/1567205013666160930110551
Prasad S, Sajja RK, Naik P, Cucullo L (2014) Diabetes mellitus and blood–brain barrier dysfunction: an overview. J Pharmacovigil 2:125. https://doi.org/10.4172/2329-6887.1000125
Acharya NK, Levin EC, Clifford PM, Han M, Tourtellotte R, Chamberlain D, Coretti NJ, Kosciuk MC, Nagele EP, DeMarshall C (2013) Diabetes and hypercholesterolemia increase blood–brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J Alzheimer’s Dis 35:179–198. https://doi.org/10.3233/JAD-122254
Foley P (2010) Lipids in Alzheimer’s disease: a century-old story. Biochim Biophys Acta Mol Cell Biol Lipids 1801:750–753. https://doi.org/10.1002/hipo.20548
Liu Q, Zhang J (2014) Lipid metabolism in Alzheimer’s disease. Neurosci Bull 30:331–345
Kurek K, Wiesiołek-Kurek P, Piotrowska DM, Łukaszuk B, Chabowski A, Żendzian-Piotrowska M (2014) Inhibition of ceramide de novo synthesis with myriocin affects lipid metabolism in the liver of rats with streptozotocin-induced type 1 diabetes. BioMed Res Int. https://doi.org/10.1155/2014/980815
Lim WL, Martins IJ, Martins RN (2014) The involvement of lipids in Alzheimer’s disease. J Genet Genomics 41:261–274. https://doi.org/10.1016/j.jgg.2014.04.003
Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrancois D, Virgili J, Planel E (2014) Insulin reverses the high-fat diet-induced increase in brain A and improves memory in an animal model of Alzheimer disease. Diabetes 63:4291–4301. https://doi.org/10.2337/db14-0375
Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Rev Endocrinol 5:150–159. https://doi.org/10.1038/ncpendmet1066
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453. https://doi.org/10.1038/nm.2307
Clark A, Saad MF, Nezzer T, Uren C, Knowler WC, Bennett PH et al (1990) Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia 33:285–289. https://doi.org/10.1007/BF00403322
Pivovarova O, Höhn A, Grune T, Pfeiffer AF, Rudovich N (2016) Insulin-degrading enzyme: new therapeutic target for diabetes and Alzheimer’s disease? Ann Med 48:614–624. https://doi.org/10.1080/07853890.2016.1197416
Tundo GR, Sbardella D, Ciaccio C, Grasso G, Gioia M, Coletta A et al (2017) Multiple functions of insulin-degrading enzyme: a metabolic crosslight? Crit Rev Biochem Mol Biol 52:554–582. https://doi.org/10.1080/10409238.2017.1337707
Mahdi O, Baharuldin MTH, Nor NHM, Chiroma SM, Jagadeesan S, Moklas MAM (2019) Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed Res Ther 6:3460–3484. https://doi.org/10.15419/bmrat.v6i11.575
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98:2133–2223. https://doi.org/10.1152/physrev.00063.2017
Bedse G, Di Domenico F, Serviddio G, Cassano T (2015) Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front Neurosci 9:204. https://doi.org/10.3389/fnins.2015.00204
Tumminia A, Vinciguerra F, Parisi M, Frittitta L (2018) Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signaling and therapeutic implications. Int J Mol Sci 19:3306. https://doi.org/10.3390/ijms19113306
Yang Y, Wu Y, Zhang S, Song W (2013) High glucose promotes Aβ production by inhibiting APP degradation. PLoS ONE 8:503. https://doi.org/10.1016/j.biopha.2018.12.133
Devi L, Alldred MJ, Ginsberg SD, Ohno M (2012) Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s disease. PLoS ONE 7:e32792. https://doi.org/10.1371/journal.pone.0032792
Lin F, Jia J, Qin W (2014) Enhancement of β-amyloid oligomer accumulation after intracerebroventricular injection of streptozotocin, which involves central insulin signaling in a transgenic mouse model. NeuroReport 25:1289–1295. https://doi.org/10.1097/WNR.0000000000000261
Liu Y, Liu L, Lu S, Wang D, Liu XD, Xie L et al (2011) Impaired amyloid β-degrading enzymes in brain of streptozotocin-induced diabetic rats. J Endocrinol Investig 34:26–31. https://doi.org/10.1007/BF03346691
Dra LA, Sellami S, Rais H, Aziz F, Aghraz A, Bekkouche K (2019) Antidiabetic potential of Caralluma europaea against alloxan-induced diabetes in mice. Saudi J Biol Sci 26:1171–1178. https://doi.org/10.1016/j.sjbs.2018.05.028
Min AY, Yoo JM, Sok DE, Kim MR (2020) Mulberry fruit prevents diabetes and diabetic dementia by regulation of blood glucose through upregulation of antioxidative activities and CREB/BDNF pathway in alloxan-induced diabetic mice. Oxid Med Cell Longev. https://doi.org/10.1155/2020/1298691
Baldissera MD, Souza CF, Grando TH, Sagrillo MR, da Silva AS, Stefani LM et al (2017) The use of tucumã oil (Astrocaryum vulgare) in alloxan-induced diabetic mice: effects on behavior, oxidant/antioxidant status, and enzymes involved in brain neurotransmission. Mol Cell Biochem 436:159–166. https://doi.org/10.1007/s11010-017-3087-9
Pradhan SP, Sahoo S, Behera A, Sahoo R, Sahu PK (2022) Memory amelioration by hesperidin conjugated gold nanoparticles in diabetes induced cognitive impaired rats. J Drug Deliv Sci Technol 69:103145. https://doi.org/10.1016/j.jddst.2022.103145
Francisqueti FV, Santos KC, Ferron AJ, Lo AT, Minatel IO, Campos DH (2016) Fructose: toxic effect on cardiorenal risk factors and redox state. SAGE Open Med 4:2050312116684294. https://doi.org/10.1177/2050312116684294
Takechi R, Lam V, Brook E, Giles C, Fimognari N, Mooranian A et al (2017) Blood–brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: an implication for causal link. Front Aging Neurosci 9:399. https://doi.org/10.3389/fnagi.2017.00399
Yabe E, Yamakawa S, Tomari HS, Kintaka Y, Uehara Y (2018) Fructose-induced cognitive dysfunction is associated with increased oxidative stress in the rat brains. J Biosci Med 6:52–64
Johnson RJ, Gomez-Pinilla F, Nagel M, Nakagawa T, Rodriguez-Iturbe B, Sanchez-Lozada LG (2020) Cerebral fructose metabolism as a potential mechanism driving Alzheimer’s disease. Front Aging Neurosci 12:299. https://doi.org/10.3389/fnagi.2020.560865
Cisternas P, Salazar P, Serrano FG, Montecinos-Oliva C, Arredondo SB, Varela-Nallar L (2015) Fructose consumption reduces hippocampal synaptic plasticity underlying cognitive performance. Biochim Biophys Acta Mol Basis Dis 1852:2379–2390. https://doi.org/10.1016/j.bbadis.2015.08.016
Hasanein P, Seifi R, Hajinezhad MR, Emamjomeh A (2016) Rosmarinic acid protects against chronic ethanol-induced learning and memory deficits in rats. Nutr Neurosci 20:547–554. https://doi.org/10.1080/1028415X.2016.1203125
Amrapali M, Sweta G, Dharmendra M, Rahul T, Shaktipal P (2020) Ethanol-induced oxidative stress and cognitive impairment in rats: neuroprotective effects of silymarin as a flavonoids. Int J Adv Sci Technol 29:8915–8927. http://sersc.org/journals/index.php/IJAST/article/view/35566
Patil S, Tawari S, Mundhada D, Nadeem S (2015) Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol Biochem Behav 136:13–20. https://doi.org/10.1016/j.pbb.2015.07.001
Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D et al (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA 107:7036–7041. https://doi.org/10.1073/pnas.1000645107
Nagai N, Ito Y, Tanino T (2015) Effect of high glucose levels on amyloid β production in retinas of spontaneous diabetes mellitus Otsuka Long-Evans Tokushima fatty rats. Biol Pharm Bull 38:601–610. https://doi.org/10.1248/bpb.b14-00819
Ajayi AM, John KA, Emmanuel IB, Chidebe EO, Adedapo AD (2021) High-fat diet-induced memory impairment and anxiety-like behavior in rats attenuated by peel extract of Ananas comosus fruit via atheroprotective, antioxidant and anti-inflammatory actions. Metabol Open 9:100077. https://doi.org/10.1016/j.metop.2021.100077
Rudrapal M, Khairnar JS, Jadhav GA (2020) Drug repurposing (DR): an emerging approach in drug discovery. In: Drug repurposing hypothesis molecular aspects and therapeutic applications. IntechOpen, London
Jourdan JP, Bureau R, Rochais C, Dallemagne P (2020) Drug repositioning: a brief overview. J Pharm Pharmacol 72:1145–1151. https://doi.org/10.1111/jphp.13273
Haj-Ali V, Mohaddes G, Babri SH (2009) Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci 123:1309–1314
Renner DB, Svitak AL, Gallus NJ, Ericson ME, Frey WH, Hanson LR (2012) Intranasal delivery of insulin via the olfactory nerve pathway. J Pharm Pharmacol 64:1709–1714. https://doi.org/10.1111/j.2042-7158.2012.01555.x
Drejer K, Vaag A, Bech K, Hansen P, Sørensen AR, Mygind N (1992) Intranasal administration of insulin with phospholipid as absorption enhancer: pharmacokinetics in normal subjects. Diabet Med 9:335–340. https://doi.org/10.1111/j.1464-5491.1992.tb01792.x
Freiherr J, Hallschmid M, Frey WH, Brünner YF, Chapman CD, Hölscher C et al (2013) Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 27:505–514. https://doi.org/10.1007/s40263-013-0076-8
Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z et al (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363. https://doi.org/10.1016/j.bbi.2017.12.009
Chen JL, Luo C, Pu D, Zhang GQ, Zhao YX, Sun Y et al (2019) Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Exp Neurol 311:44–56. https://doi.org/10.1016/j.expneurol.2018.09.008
Wang Y, Zhao J, Guo FL, Gao X, Xie X, Liu S, Yang X, Zhang L, Ye Y, Fan L (2020) Metformin ameliorates synaptic defects in a mouse model of AD by inhibiting Cdk5 activity. Front Cell Neurosci 14:170. https://doi.org/10.3389/fncel.2020.00170
Ma X, Xiao W, Li H, Pang P, Xue F, Wan L, Pei L, Yan H (2021) Metformin restores hippocampal neurogenesis and learning and memory via regulating gut microbiota in the obese mouse model. Brain Behav Immun 95:68–83. https://doi.org/10.1016/j.bbi.2021.02.011
Chen Y, Zhao S, Fan Z, Li Z, Zhu Y, Shen T, Li K, Yan Y, Tian J, Liu Z, Zhang B (2021) Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice. Alzheimer’s Res Ther 13:1–3. https://doi.org/10.1186/s13195-020-00761-9
Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, Baggiella E (2016) Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimer’s Dis 1:501–514. https://doi.org/10.3233/jad-150493
Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L et al (2017) Effects of the insulin sensitizer metformin in Alzheimer’s disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer’s Dis Assoc Disord 31:107–113. https://doi.org/10.1097/WAD.0000000000000202
Shi Q, Liu S, Fonseca VA, Thethi TK, Shi L (2019) Effect of metformin on neurodegenerative disease among elderly adult US Veterans with type 2 diabetes mellitus. BMJ Open 9:e024954. https://doi.org/10.1136/bmjopen-2018-024954
Asadbegi M, Yaghmaei P, Salehi I, Ebrahim-Habibi A, Komaki A (2016) Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res Bull 121:178–185. https://doi.org/10.1016/j.brainresbull.2016.02.005
Li J, Deng J, Sheng W, Zuo Z (2012) Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav 101:564–574. https://doi.org/10.1016/j.pbb.2012.03.002
Alp H, Varol S, Celik MM, Altas M, Evliyaoglu O, Tokgoz O et al (2012) Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozotocin. Exp Diabetes Res. https://doi.org/10.1155/2012/230342
Baraka A, ElGhotny S (2010) Study of the effect of inhibiting galanin in Alzheimer’s disease induced in rats. Eur J Pharmacol 641:123–127. https://doi.org/10.1016/j.ejphar.2010.05.030
Patel AD, Gerzanich V, Geng Z, Simard JM (2010) Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol 69:1177–1190. https://doi.org/10.1097/NEN.0b013e3181fbf6d6
Tosun C, Kurland DB, Mehta R, Castellani RJ, deJong JL, Kwon MS et al (2013) Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 44:3522–3528. https://doi.org/10.1161/STROKEAHA.113.002904
Luo D, Hou X, Hou L, Wang M, Xu S, Dong C et al (2011) Effect of pioglitazone on altered expression of Aβ metabolism-associated molecules in the brain of fructose-drinking rats, a rodent model of insulin resistance. Eur J Pharmacol 664:14–19. https://doi.org/10.3233/jad-150493
Gad ES, Zaitone SA, Moustafa YM (2016) Pioglitazone and exenatide enhance cognition and downregulate hippocampal beta amyloid oligomer and microglia expression in insulin-resistant rats. Can J Physiol Pharmacol 94:819–828. https://doi.org/10.1139/cjpp-2015-0242
Assaf N, El-Shamarka ME, Salem NA, Khadrawy YA, El Sayed NS (2020) Neuroprotective effect of PPAR alpha and gamma agonists in a mouse model of amyloidogenesis through modulation of the Wnt/beta catenin pathway via targeting alpha-and beta-secretases. Prog Neuropsychopharmacol Biol Psychiatry 97:109793. https://doi.org/10.1016/j.pnpbp.2019.109793
Hu SH, Jiang T, Yang SS, Yang Y (2013) Pioglitazone ameliorates intracerebral insulin resistance and tau-protein hyperphosphorylation in rats with type 2 diabetes. Exp Clin Endocrinol Diabetes 121:220–224. https://doi.org/10.1055/s-0032-1333277
Jiang LY, Tang SS, Wang XY, Liu LP, Long Y, Hu M et al (2012) PPARγ agonist pioglitazone reverses memory impairment and biochemical changes in a mouse model of type 2 diabetes mellitus. CNS Neurosci Ther 18:659–666. https://doi.org/10.1111/j.1755-5949.2012.00341.x
Yang S, Chen Z, Cao M, Li R, Wang Z, Zhang M (2017) Pioglitazone ameliorates Aβ42 deposition in rats with diet-induced insulin resistance associated with AKT/GSK3β activation. Mol Med Rep 15:2588–2594. https://doi.org/10.3892/mmr.2017.6342
Yin QQ, Pei JJ, Xu S, Luo DZ, Dong SQ, Sun MH et al (2013) Pioglitazone improves cognitive function via increasing insulin sensitivity and strengthening antioxidant defense system in fructose-drinking insulin resistance rats. PLoS ONE 8:e59313. https://doi.org/10.1371/journal.pone.0059313
Toba J, Nikkuni M, Ishizeki M, Yoshii A, Watamura N, Inoue T et al (2016) PPARγ agonist pioglitazone improves cerebellar dysfunction at pre-Aβ deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem Biophys Res Commun 473:1039–1044. https://doi.org/10.1016/j.bbrc.2016.04.012
Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F et al (2015) Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm 122:593–606. https://doi.org/10.1007/s00702-014-1294-z
Wang H, Chen F, Zhong KL, Tang SS, Hu M, Long Y et al (2016) PPARγ agonists regulate bidirectional transport of amyloid-β across the blood–brain barrier and hippocampus plasticity in db/db mice. Br J Pharmacol 173:372–385. https://doi.org/10.1111/bph.13378
Hsu WC, Wildburger NC, Haidacher SJ, Nenov MN, Folorunso O, Singh AK et al (2017) PPARgamma agonists rescue increased phosphorylation of FGF14 at S226 in the Tg2576 mouse model of Alzheimer’s disease. Exp Neurol 295:1–7. https://doi.org/10.1016/j.expneurol.2017.05.005
Hunter K, Hölscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood–brain barrier and enhance neurogenesis. BMC Neurosci 13:1–6. https://doi.org/10.1186/1471-2202-13-33
Paladugu L, Gharaibeh A, Kolli N, Learman C, Hall TC, Li L et al (2021) Liraglutide has anti-inflammatory and anti-amyloid properties in streptozotocin-induced and 5xFAD mouse models of Alzheimer’s disease. Int J Mol Sci 22:860. https://doi.org/10.3390/ijms22020860
Palleria C, Leo A, Andreozzi F, Citraro R, Iannone M, Spiga R et al (2017) Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav Brain Res 321:157–169. https://doi.org/10.1016/j.bbr.2017.01.004
Qi L, Ke L, Liu X, Liao L, Ke S, Liu X et al (2016) Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced Alzheimer disease mouse model. Eur J Pharmacol 783:23–32. https://doi.org/10.1016/j.ejphar.2016.04.052
Cai HY, Hölscher C, Yue XH, Zhang SX, Wang XH, Qiao F, Yang W, Qi JS (2014) Lixisenatide rescues spatial memory and synaptic plasticity from amyloid β protein-induced impairments in rats. Neuroscience 277:6–13. https://doi.org/10.1016/j.neuroscience.2014.02.022
Watson KT, Wroolie TE, Tong G, Foland-Ross LC, Frangou S, Singh M, McIntyre RS, Roat-Shumway S, Myoraku A, Reiss AL, Rasgon NL (2019) Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav Brain Res 356:271–278. https://doi.org/10.1016/j.bbr.2018.08.006
Solmaz V, Çınar BP, Yiğittürk G, Çavuşoğlu T, Taşkıran D, Erbaş O (2015) Exenatide reduces TNF-α expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. Eur J Pharmacol 765:482–487. https://doi.org/10.1016/j.ejphar.2015.09.024
Gumuslu E, Mutlu O, Celikyurt IK, Ulak G, Akar F, Erden F (2016) Exenatide enhances cognitive performance and upregulates neurotrophic factor gene expression levels in diabetic mice. Fundam Clin Pharmacol 30:376–384. https://doi.org/10.1111/fcp.12192
An J, Zhou Y, Zhang M, Xie Y, Ke S, Liu L (2016) Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5× FAD mouse model of Alzheimer’s disease. Behav Brain Res 370:111932. https://doi.org/10.1016/j.bbr.2019.111932
Bomba M, Granzotto A, Castelli V, Onofrj M, Lattanzio R, Cimini A et al (2017) Exenatide reverts the high-fat-diet-induced impairment of BDNF signaling and inflammatory response in an animal model of Alzheimer’s disease. J Alzheimer’s Dis 70:793–810. https://doi.org/10.3233/jad-190237
Zhou M, Chen S, Peng P, Gu Z, Yu J, Zhao G et al (2019) Dulaglutide ameliorates STZ induced AD-like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3β. Biochem Biophys Res Commun 511:154–160. https://doi.org/10.1016/j.bbrc.2019.01.103
Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK et al (2013) Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol 65:1773–1784. https://doi.org/10.1111/jphp.12148
Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2013) DPP 4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci 37:839–849. https://doi.org/10.1111/ejn.12088
Sripetchwandee J, Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2014) DPP-4 inhibitor and PPARγ agonist restore the loss of CA1 dendritic spines in obese insulin-resistant rats. Arch Med Res 45:547–552. https://doi.org/10.1016/j.arcmed.2014.09.002
Pintana H, Tanajak P, Pratchayasakul W, Sa-Nguanmoo P, Chunchai T, Satjaritanun P et al (2016) Energy restriction combined with dipeptidyl peptidase-4 inhibitor exerts neuroprotection in obese male rats. Br J Nutr 116:1700–1708. https://doi.org/10.1017/S0007114516003871
Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RD et al (2013) Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology 72:291–300. https://doi.org/10.1016/j.neuropharm.2013.04.008
Kosaraju J, Holsinger RD, Guo L, Tam KY (2017) Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Mol Neurobiol 54:6074–6084. https://doi.org/10.1007/s12035-016-0125-7
Gault VA, Lennox R, Flatt PR (2015) Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab 17:403–413. https://doi.org/10.1111/dom.12432
Isik AT, Soysal P, Yay A, Usarel C (2017) The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract 123:192–198. https://doi.org/10.1016/j.diabres.2016.12.010
Hierro-Bujalance C, Infante-Garcia C, Del Marco A, Herrera M, Carranza-Naval MJ, Suarez J et al (2020) Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimer’s Res Ther 12:1–3. https://doi.org/10.1186/s13195-020-00607-4
Son SM, Jung ES, Shin HJ, Byun J, Mook-Jung I (2012) Aβ-induced formation of autophagosomes is mediated by RAGE-CaMKKβ-AMPK signaling. Neurobiol Aging 33:1006-e11-23. https://doi.org/10.1016/j.neurobiolaging.2011.09.039
Baradaran Z, Vakilian A, Zare M, Hashemzehi M, Hosseini M, Dinpanah H et al (2021) Metformin improved memory impairment caused by chronic ethanol consumption during adolescent to adult period of rats: role of oxidative stress and neuroinflammation. Behav Brain Res 1:113399. https://doi.org/10.1016/j.bbr.2021.113399
Jantrapirom S, Nimlamool W, Chattipakorn N, Chattipakorn S, Temviriyanukul P, Inthachat W (2020) Liraglutide suppresses tau hyperphosphorylation, amyloid beta accumulation through regulating neuronal insulin signaling and BACE-1 activity. Int J Mol Sci 21:1725. https://doi.org/10.3390/ijms21051725
Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Jaiwongkam T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2017) SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol 333:43–50. https://doi.org/10.1016/j.taap.2017.08.005
Lu XY, Huang S, Chen QB, Zhang D, Li W, Ao R et al (2020) Metformin ameliorates Aβ pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer’s disease. Oxid Med Cell Longev 2020:2315106
Liu LP, Yan TH, Jiang LY, Hu W, Hu M, Wang C et al (2013) Pioglitazone ameliorates memory deficits in streptozotocin-induced diabetic mice by reducing brain β-amyloid through PPARγ activation. Acta Pharmacol Sin 34:455–463
Chen S, Tang Q, Wang Y, Xu Z, Chen ST, Sun Y et al (2019) Evidence of metabolic memory-induced neurodegeneration and the therapeutic effects of glucagon-like peptide-1 receptor agonists via Forkhead box class O. Biochim Biophys Acta Mol Basis Dis 1865:371–377. https://doi.org/10.1016/j.bbadis.2018.11.012
Łabuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopień B et al (2010) Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 6:956–965. https://doi.org/10.1016/S1734-1140(10)70357-1
Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R et al (2014) Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 35:793–801. https://doi.org/10.1016/j.neurobiolaging.2013.10.076
Festuccia WT, Oztezcan S, Laplante M, Berthiaume M, Michel C, Dohgu S et al (2008) Peroxisome proliferator-activated receptor-γ-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology 149:2121–2130. https://doi.org/10.1210/en.2007-1553
Hunter K, Hölscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood–brain barrier and enhance neurogenesis. BMC Neurosci 13:33. https://doi.org/10.1186/1471-2202-13-33
Dong M, Wen S, Zhou L (2022) The relationship between the blood–brain-barrier and the central effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors. Diabetes Metab Syndr Obes Target Ther 15:2583. https://doi.org/10.2147/DMSO.S375559
Kastin AJ, Akerstrom V (2003) Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27:313–318. https://doi.org/10.1038/sj.ijo.0802206
Akimoto H, Negishi A, Oshima S, Wakiyama H, Okita M, Horii N et al (2020) Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. Am J Alzheimer’s Dis Dement 35:1533317519899546. https://doi.org/10.1177/1533317519899546
Wang X, Chang Y, He Y, Lyu C, Li H, Zhu J (2020) Glimepiride and glibenclamide have comparable efficacy in treating acute ischemic stroke in mice. Neuropharmacology 162:107845. https://doi.org/10.1016/j.neuropharm.2019.107845
Zhang DD, Shi N, Fang H, Ma L, Wu WP, Zhang YZLB (2018) Vildagliptin, a DPP4 inhibitor, alleviates diabetes-associated cognitive deficits by decreasing the levels of apoptosis-related proteins in the rat hippocampus. Exp Ther Med 15:5100–5106. https://doi.org/10.3892/etm.2018
Kesharwani P, Gorain B, Low SY, Tan SA, Ling EC, Lim YK et al (2018) Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res Clin Pract 136:52–77
Arora D, Jaglan S (2018) Therapeutic applications of resveratrol nanoformulations. Environ Chem Lett 16:35–41. https://doi.org/10.1007/s10311-017-0660-0
Behera A, Padhi S (2020) Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01022-9
Patra JK, Das G, Fraceto LF, Campos EV, del Pilar R-T, Acosta-Torres LS et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16:1–33. https://doi.org/10.1186/s12951-018-0392-8
Aryaeian N, Sedehi SK, Arablou T (2017) Polyphenols and their effects on diabetes management: a review. Med J Islam Repub Iran 31:134. https://doi.org/10.14196/mjiri.31.134
Khurana RK, Gaspar BL, Welsby G, Katare OP, Singh KK, Singh B (2018) Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv Transl Res 8:617–632. https://doi.org/10.1007/s13346-018-0498-4
Sharma M, Sharma R, Jain DK (2016) Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica. https://doi.org/10.1155/2016/8525679
Saka R, Chella N (2021) Nanotechnology for delivery of natural therapeutic substances: a review. Environ Chem Lett 19:1097–1106. https://doi.org/10.1007/s10311-020-01103-9
Sengul AB, Asmatulu E (2020) Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett 18:1659–1683. https://doi.org/10.1007/s10311-020-01033-6
Meena J, Gupta A, Ahuja R, Singh M, Bhaskar S, Panda A (2022) Inorganic nanoparticles for natural product delivery: a review. Environ Chem Lett 18:2107–2118. https://doi.org/10.1007/s10311-020-01061-2
Hua S, Wu SY (2013) The use of lipid-based nanocarriers for targeted pain therapies. Front Pharmacol 4:143. https://doi.org/10.3389/fphar.2013.00143
Barçin Z (2014) Anti-Parkinsonian drug delivery across the blood–brain barrier. Master's Thesis, Middle East Technical University
Salunkhe SS, Bhatia NM, Kawade VS, Bhatia MS (2015) Development of lipid based nanoparticulate drug delivery systems and drug carrier complexes for delivery to brain. J Appl Pharm Sci 5:110–129. https://doi.org/10.7324/JAPS.2015.50521
Yadav A, Ghune M, Jain DK (2011) Nano-medicine based drug delivery system. J Adv Pharm Educ Res 1:201–213
Guo S, Huang L (2014) Nanoparticles containing insoluble drug for cancer therapy. Biotechnol Adv 32:778–788. https://doi.org/10.1016/j.biotechadv.2013.10.002
Xu W, Ling P, Zhang T (2013) Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. https://doi.org/10.1155/2013/340315
Ahmad Z, Shah A, Siddiq M, Kraatz HB (2014) Polymeric micelles as drug delivery vehicles. RSC Adv 4:17028–17038. https://doi.org/10.1039/C3RA47370H
Choudhary S, Gupta L, Rani S, Dave K, Gupta U (2017) Impact of dendrimers on solubility of hydrophobic drug molecules. Front Pharmacol 8:261. https://doi.org/10.3389/fphar.2017.00261
Saeedi M, Eslamifar M, Khezri K, Dizaj SM (2019) Applications of nanotechnology in drug delivery to the central nervous system. Biomed Pharmacother 111:666–675. https://doi.org/10.1016/j.biopha.2018.12.133
Shilo M, Motiei M, Hana P, Popovtzer R (2014) Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale 6:2146–2152. https://doi.org/10.1039/C3NR04878K
Betzer O, Shilo M, Opochinsky R, Barnoy E, Motiei M, Okun E et al (2017) The effect of nanoparticle size on the ability to cross the blood–brain barrier: an in vivo study. Nanomedicine 12:1533–1546. https://doi.org/10.2217/nnm-2017-0022
Picone P, Sabatino MA, Ditta LA, Amato A, San Biagio PL, Mulè F et al (2018) Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J Control Release 270:23–36. https://doi.org/10.1016/j.jconrel.2017.11.040
Hong L, Li X, Bao Y, Duvall CL, Zhang C, Chen W et al (2019) Preparation, preliminary pharmacokinetic and brain targeting study of metformin encapsulated W/O/W composite submicron emulsions promoted by borneol. Eur J Pharm Sci 133:160–166
Ebokaiwe AP, Okori S, Nwankwo JO, Ejike CE, Osawe SO (2021) Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn Schmiedebergs Arch Pharmacol 394:591–602. https://doi.org/10.1007/s00210-020-02000-2
Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F (2020) Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci 255:117861. https://doi.org/10.1016/j.lfs.2020.117861
Zeng H, Xu L, Zou Y, Wang S (2021) Romidepsin and metformin nanomaterials delivery on streptozotocin for the treatment of Alzheimer’s disease in animal model. Biomed Pharmacother 141:111864
Jojo GM, Kuppusamy G, De A, Karri VNR (2019) Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev Ind Pharm 45:1061–1072. https://doi.org/10.1080/03639045.2019.1593439
Silva-Abreu M, Espinoza LC, Halbaut L, Espina M, García ML, Calpena AC (2018) Comparative study of ex vivo transmucosal permeation of pioglitazone nanoparticles for the treatment of Alzheimer’s disease. Polymers 10:316. https://doi.org/10.3390/polym10030316
Wong LR, Wong P, Ho PC (2020) Metabolic profiling of female Tg2576 mouse brains provides novel evidence supporting intranasal low-dose pioglitazone for long-term treatment at an early stage of Alzheimer’s disease. Biomedicines 8:589. https://doi.org/10.3390/biomedicines8120589
Sarathlal KC, Kakoty V, Marathe S, Chitkara D, Taliyan R (2021) Exploring the neuroprotective potential of rosiglitazone embedded nanocarrier system on streptozotocin induced mice model of Alzheimer’s disease. Neurotox Res 39:240–255. https://doi.org/10.1007/s12640-020-00258-1
Sarathlal KC, Kakoty V, Krishna KV, Dubey SK, Chitkara D, Taliyan R (2021) Neuroprotective efficacy of co-encapsulated rosiglitazone and vorinostat nanoparticle on streptozotocin induced mice model of Alzheimer disease. ACS Chem Neurosci 12:1528–1541. https://doi.org/10.1021/acschemneuro.1c00022
Wilson B, Alobaid BNM, Geetha KM, Jenita JL (2021) Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J Drug Deliv Sci Technol 61:102176. https://doi.org/10.1016/j.jddst.2020.102176
Fernandes J, Ghate MV, Mallik SB, Lewis SA (2018) Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int J Pharm 547:563–571. https://doi.org/10.1016/j.ijpharm.2018.06.031
Khan T, Khan S, Akhtar M, Ali J, Najmi AK (2021) Empagliflozin nanoparticles attenuates type 2 diabetes induced cognitive impairment via oxidative stress and inflammatory pathway in high fructose diet induced hyperglycemic mice. Neurochem Int 150:105158. https://doi.org/10.1016/j.neuint.2021.105158
Acknowledgements
The authors are thankful for the e-library facility provided by the Siksha ‘O’ Anusandhan Deemed to be University for the preparation of this review article
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Sweta Priyadarshini Pradhan - Wrote the main manuscript and prepared the figures 1 and 2 Pratap Kumar Sahu and Anindita Behera - Done the final review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pradhan, S.P., Sahu, P.K. & Behera, A. New insights toward molecular and nanotechnological approaches to antidiabetic agents for Alzheimer’s disease. Mol Cell Biochem 478, 2739–2762 (2023). https://doi.org/10.1007/s11010-023-04696-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04696-1