Abstract
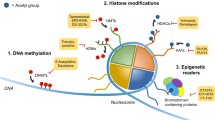
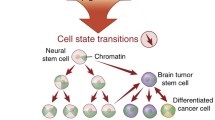
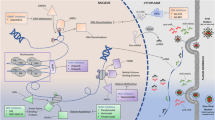
Medulloblastoma, neuroblastoma, and pediatric glioma account for almost 30% of all cases of pediatric cancers. Recent evidence indicates that pediatric nervous system tumors originate from stem or progenitor cells and present a subpopulation of cells with highly tumorigenic and stem cell-like features. These cancer stem cells play a role in initiation, progression, and resistance to treatment of pediatric nervous system tumors. Histone modification, DNA methylation, chromatin remodeling, and microRNA regulation display a range of regulatory activities involved in cancer origin and progression, and cellular identity, especially those associated with stem cell features, such as self-renewal and pluripotent differentiation potential. Here, we review the contribution of different epigenetic mechanisms in pediatric nervous system tumor cancer stem cells. The choice between a differentiated and undifferentiated state can be modulated by alterations in the epigenome through the regulation of stemness genes such as CD133, SOX2, and BMI1 and the activation neuronal of differentiation markers, RBFOX3, GFAP, and S100B. Additionally, we highlighted the stage of development of epigenetic drugs and the clinical benefits and efficacy of epigenetic modulators in pediatric nervous system tumors.

Similar content being viewed by others
Data availability
Not applicable.
References
Steliarova-Foucher E, Colombet M, Ries LAG et al (2017) International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol 18:719–731. https://doi.org/10.1016/S1470-2045(17)30186-9
Ma X, Liu Y, Liu Y et al (2018) Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 555:371–376. https://doi.org/10.1038/nature25795
Gröbner SN, Worst BC, Weischenfeldt J et al (2018) The landscape of genomic alterations across childhood cancers. Nature 555:321–327. https://doi.org/10.1038/nature25480
Downing JR, Wilson RK, Zhang J et al (2012) The pediatric cancer genome project. Nat Genet 44:619–622. https://doi.org/10.1038/ng.2287
Huether R, Dong L, Chen X et al (2014) The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat Commun. https://doi.org/10.1038/ncomms4630
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Qureshi IA, Mehler MF (2011) Epigenetics, nervous system tumors, and cancer stem cells. Cancers 3:3525–3556. https://doi.org/10.3390/cancers3033525
Tomolonis JA, Agarwal S, Shohet JM (2017) Neuroblastoma pathogenesis: deregulation of embryonic neural crest development. Cell Tissue Res 372:245–262. https://doi.org/10.1007/s00441-017-2747-0
Lu QR, Qian L, Zhou X (2019) Developmental origins and oncogenic pathways in malignant brain tumors. Wiley Interdiscip Rev. https://doi.org/10.1002/wdev.342
Han JW, Yoon Y-S (2012) Epigenetic landscape of pluripotent stem cells. Antioxid Redox Signal 17:205–223. https://doi.org/10.1089/ars.2011.4375
Hemmati HD, Nakano I, Lazareff JA et al (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci 100:15178–15183. https://doi.org/10.1073/pnas.2036535100
Walton JD, Kattan DR, Thomas SK et al (2004) Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia 6:838–845. https://doi.org/10.1593/neo.04310
Thirant C, Bessette B, Varlet P et al (2011) Clinical relevance of tumor cells with stem-like properties in pediatric brain tumors. PLoS ONE. https://doi.org/10.1371/journal.pone.0016375
Northcott PA, Robinson GW, Kratz CP et al (2019) Medulloblastoma. Nat Rev Dis Primers. https://doi.org/10.1038/s41572-019-0063-6
Johnsen JI, Dyberg C, Wickström M (2019) Neuroblastoma—a neural crest derived embryonal malignancy. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2019.00009
Funakoshi Y, Hata N, Kuga D et al (2021) Pediatric glioma: an update of diagnosis, biology, and treatment. Cancers 13:758. https://doi.org/10.3390/cancers13040758
Lawlor ER, Thiele CJ (2012) Epigenetic changes in pediatric solid tumors: promising new targets. Clin Cancer Res 18:2768–2779. https://doi.org/10.1158/1078-0432.CCR-11-1921
Franco SS, Szczesna K, Iliou MS et al (2016) In vitro models of cancer stem cells and clinical applications. BMC Cancer. https://doi.org/10.1186/s12885-016-2774-3
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. https://doi.org/10.1038/nature03128
Ross RA, Walton JD, Han D et al (2015) A distinct gene expression signature characterizes human neuroblastoma cancer stem cells. Stem Cell Res 15:419–426. https://doi.org/10.1016/j.scr.2015.08.008
Pandian V, Ramraj S, Khan FH et al (2015) Metastatic neuroblastoma cancer stem cells exhibit flexible plasticity and adaptive stemness signaling. Stem Cell Res Ther. https://doi.org/10.1186/s13287-015-0002-8
Nightingale KP, O’Neill LP, Turner BM (2006) Histone modifications: signalling receptors and potential elements of a heritable epigenetic code. Curr Opin Genet Dev 16:125–136. https://doi.org/10.1016/j.gde.2006.02.015
Yang X-J, Seto E (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26:5310–5318. https://doi.org/10.1038/sj.onc.1210599
Liu N, Li S, Wu N, Cho K-S (2017) Acetylation and deacetylation in cancer stem-like cells. Oncotarget 8:89315–89325. https://doi.org/10.18632/oncotarget.19167
Nör C, Sassi FA, Farias CBD et al (2013) The histone deacetylase inhibitor sodium butyrate promotes cell death and differentiation and reduces neurosphere formation in human medulloblastoma cells. Mol Neurobiol 48:533–543. https://doi.org/10.1007/s12035-013-8441-7
Jaeger MDC, Ghisleni EC, Cardoso PS et al (2020) HDAC and MAPK/ERK inhibitors cooperate to reduce viability and stemness in medulloblastoma. J Mol Neurosci 70:981–992. https://doi.org/10.1007/s12031-020-01505-y
Yuan J, Luceño NL, Sander B, Golas MM (2017) Synergistic anti-cancer effects of epigenetic drugs on medulloblastoma cells. Cell Oncol 40:263–279. https://doi.org/10.1007/s13402-017-0319-7
Almeida VR, Vieira IA, Buendia M et al (2016) Combined treatments with a retinoid receptor agonist and epigenetic modulators in human neuroblastoma cells. Mol Neurobiol 54:7610–7619. https://doi.org/10.1007/s12035-016-0250-3
Chateauvieux S, Morceau F, Dicato M, Diederich M (2010) Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol 2010:1–18. https://doi.org/10.1155/2010/479364
Stockhausen M-T, Sjölund J, Manetopoulos C, Axelson H (2005) Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer 92:751–759. https://doi.org/10.1038/sj.bjc.6602309
Khalil MA, Hraběta J, Groh T et al (2016) Valproic acid increases CD133 positive cells that show low sensitivity to cytostatics in neuroblastoma. PLoS ONE. https://doi.org/10.1371/journal.pone.0162916
Hahn CK, Ross KN, Warrington IM et al (2008) Expression-based screening identifies the combination of histone deacetylase inhibitors and retinoids for neuroblastoma differentiation. Proc Natl Acad Sci 105:9751–9756. https://doi.org/10.1073/pnas.0710413105
Zheng X, Naiditch J, Czurylo M et al (2013) Differential effect of long-term drug selection with doxorubicin and vorinostat on neuroblastoma cells with cancer stem cell characteristics. Cell Death Dis. https://doi.org/10.1038/cddis.2013.264
Tsui, M. K. H (2009) Histone deacetylase inhibitor MS-275 inhibits neuroblastoma cell growth by inducing cell cycle arrest, apoptosis, differentiation and by targeting its tumor stem cell population. Thesis, University of Toronto
Mokhtari RB, Baluch N, Tsui MKH et al (2017) Acetazolamide potentiates the anti-tumor potential of HDACi, MS-275, in neuroblastoma. BMC Cancer. https://doi.org/10.1186/s12885-017-3126-7
Wegener D, Deubzer HE, Oehme I et al (2008) HKI 46F08, a novel potent histone deacetylase inhibitor, exhibits antitumoral activity against embryonic childhood cancer cells. Anticancer Drugs 19:849–857. https://doi.org/10.1097/cad.0b013e32830efbeb
Oehme I, Deubzer HE, Wegener D et al (2008) Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin Cancer Res 15:91–99. https://doi.org/10.1158/1078-0432.CCR-08-0684
Rettig I, Koeneke E, Trippel F et al (2015) Selective inhibition of HDAC8 decreases neuroblastoma growth in vitro and in vivo and enhances retinoic acid-mediated differentiation. Cell Death Dis. https://doi.org/10.1038/cddis.2015.24
Frumm SM, Fan ZP, Ross KN et al (2013) Selective HDAC1/HDAC2 Inhibitors Induce neuroblastoma differentiation. Chem Biol 20:713–725. https://doi.org/10.1016/j.chembiol.2013.03.020
Anastas JN, Zee BM, Kalin JH et al (2019) Re-programing chromatin with a bifunctional LSD1/HDAC inhibitor induces therapeutic differentiation in DIPG. Cancer Cell. https://doi.org/10.1016/j.ccell.2019.09.005
Meel MH, Gooijer MCD, Metselaar DS et al (2020) Combined therapy of AXL and HDAC inhibition reverses mesenchymal transition in diffuse intrinsic pontine glioma. Clin Cancer Res 26:3319–3332. https://doi.org/10.1158/1078-0432.CCR-19-3538
Grasso CS, Tang Y, Truffaux N et al (2015) Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 21:555–559. https://doi.org/10.1038/nm.3855
Pötschke R, Gielen G, Pietsch T et al (2019) Musashi1 enhances chemotherapy resistance of pediatric glioblastoma cells in vitro. Pediatr Res 87:669–676. https://doi.org/10.1038/s41390-019-0628-9
Barski A, Cuddapah S, Cui K et al (2007) High-resolution profiling of histone methylations in the human genome. Cell 129:823–837. https://doi.org/10.1016/j.cell.2007.05.009
Wainwright EN, Scaffidi P (2017) Epigenetics and cancer stem cells: unleashing, hijacking, and restricting cellular plasticity. Trends in Cancer 3:372–386. https://doi.org/10.1016/j.trecan.2017.04.004
Wen Y, Cai J, Hou Y et al (2017) Role of EZH2 in cancer stem cells: from biological insight to a therapeutic target. Oncotarget 8:37974–37990. https://doi.org/10.18632/oncotarget.16467
Miele E, Valente S, Alfano V et al (2017) The histone methyltransferase EZH2 as a druggable target in SHH medulloblastoma cancer stem cells. Oncotarget 8:68557–68570. https://doi.org/10.18632/oncotarget.19782
Alimova I, Venkataraman S, Harris P et al (2012) Targeting the enhancer of zeste homologue 2 in medulloblastoma. Int J Cancer 131:1800–1809. https://doi.org/10.1002/ijc.27455
Liu H, Sun Q, Sun Y et al (2017) MELK and EZH2 cooperate to regulate medulloblastoma cancer stem-like cell proliferation and differentiation. Mol Cancer Res 15:1275–1286. https://doi.org/10.1158/1541-7786.MCR-17-0105
Wang C, Liu Z, Woo C-W et al (2011) EZH2 mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Can Res 72:315–324. https://doi.org/10.1158/0008-5472.CAN-11-0961
Schulte JH, Lim S, Schramm A et al (2009) Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Can Res 69:2065–2071. https://doi.org/10.1158/0008-5472.CAN-08-1735
Yang J, Altahan AM, Hu D et al (2015) The role of histone demethylase KDM4B in myc signaling in neuroblastoma. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv080
Kuo Y-T, Liu Y-L, Adebayo BO et al (2015) JARID1B expression plays a critical role in chemoresistance and stem cell-like phenotype of neuroblastoma cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0125343
Lochmann TL, Powell KM, Ham J et al (2018) Targeted inhibition of histone H3K27 demethylation is effective in high-risk neuroblastoma. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao4680
Schwartzentruber J, Korshunov A, Liu X-Y et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231. https://doi.org/10.1038/nature10833
Hashizume R, Andor N, Ihara Y et al (2014) Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med 20:1394–1396. https://doi.org/10.1038/nm.3716
Lewis PW, Muller MM, Koletsky MS et al (2013) Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340:857–861. https://doi.org/10.1126/science.1232245
Bender S, Tang Y, Lindroth AM et al (2013) Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24:660–672. https://doi.org/10.1016/j.ccr.2013.10.006
Mohammad F, Weissmann S, Leblanc B et al (2017) EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat Med 23:483–492. https://doi.org/10.1038/nm.4293
Jin B, Ernst J, Tiedemann RL et al (2012) Linking DNA methyltransferases to epigenetic marks and nucleosome structure genome-wide in human tumor cells. Cell Rep 2:1411–1424. https://doi.org/10.1016/j.celrep.2012.10.017
Pfeifer G (2018) Defining driver DNA methylation changes in human cancer. Int J Mol Sci 19:1166. https://doi.org/10.3390/ijms19041166
Pechalrieu D, Etievant C, Arimondo PB (2017) DNA methyltransferase inhibitors in cancer: from pharmacology to translational studies. Biochem Pharmacol 129:1–13. https://doi.org/10.1016/j.bcp.2016.12.004
Valente S, Liu Y, Schnekenburger M et al (2014) Selective non-nucleoside inhibitors of human DNA methyltransferases active in cancer including in cancer stem cells. J Med Chem 57:701–713. https://doi.org/10.1021/jm4012627
Castresana (2010) Analysis of stemness gene expression and CD133 abnormal methylation in neuroblastoma cell lines. Oncol Rep. https://doi.org/10.3892/or_00000993
Ikegaki N, Shimada H, Fox AM et al (2013) Transient treatment with epigenetic modifiers yields stable neuroblastoma stem cells resembling aggressive large-cell neuroblastomas. Proc Natl Acad Sci 110:6097–6102. https://doi.org/10.1073/pnas.1118262110
Mackay A, Burford A, Carvalho D et al (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. https://doi.org/10.1016/j.ccell.2017.08.017
Korshunov A, Ryzhova M, Hovestadt V et al (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathologica 129:669–678. https://doi.org/10.1007/s00401-015-1405-4
Korshunov A, Schrimpf D, Ryzhova M et al (2017) H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol 134:507–516. https://doi.org/10.1007/s00401-017-1710-1
Nair SS, Kumar R (2012) Chromatin remodeling in cancer: a gateway to regulate gene transcription. Mol Oncol 6:611–619. https://doi.org/10.1016/j.molonc.2012.09.005
Robinson MH, Maximov V, Lallani S et al (2019) Upregulation of the chromatin remodeler HELLS is mediated by YAP1 in Sonic Hedgehog medulloblastoma. Sci Rep. https://doi.org/10.1038/s41598-019-50088-1
Xi S, Geiman TM, Briones V et al (2009) Lsh participates in DNA methylation and silencing of stem cell genes. Stem Cells 27:2691–2702. https://doi.org/10.1002/stem.183
Barbieri E, Preter KD, Capasso M et al (2013) Histone chaperone CHAF1A inhibits differentiation and promotes aggressive neuroblastoma. Can Res 74:765–774. https://doi.org/10.1158/0008-5472.CAN-13-1315
Klein BJ, Wang X, Cui G et al (2016) PHF20 readers link methylation of histone H3K4 and p53 with H4K16 acetylation. Cell Rep 17:1158–1170. https://doi.org/10.1016/j.celrep.2016.09.056
Long W, Zhao W, Ning B et al (2018) PHF20 collaborates with PARP1 to promote stemness and aggressiveness of neuroblastoma cells through activation of SOX2 and OCT4. J Mol Cell Biol 10:147–160. https://doi.org/10.1093/jmcb/mjy007
Green AL, Desisto J, Flannery P et al (2019) BPTF regulates growth of adult and pediatric high-grade glioma through the MYC pathway. Oncogene 39:2305–2327. https://doi.org/10.1038/s41388-019-1125-7
Lee S, Vasudevan S (2012) Post-transcriptional Stimulation of Gene Expression by MicroRNAs Advances in Experimental Medicine and Biology. Ten Years of Progress in GW/P Body Res. https://doi.org/10.1007/978-1-4614-5107-5_7
Garg N, Vijayakumar T, Bakhshinyan D et al (2015) MicroRNA regulation of brain tumour initiating cells in central nervous system tumours. Stem Cells Int 2015:1–15. https://doi.org/10.1155/2015/141793
Venkataraman S, Alimova I, Fan R et al (2010) MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS ONE. https://doi.org/10.1371/journal.pone.0010748
Venkataraman S, Birks DK, Balakrishnan I et al (2013) MicroRNA 218 acts as a tumor suppressor by targeting multiple cancer phenotype-associated genes in medulloblastoma. J Biol Chem 288:1918–1928. https://doi.org/10.1074/jbc.M112.396762
Garzia L, Andolfo I, Cusanelli E et al (2009) MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS ONE. https://doi.org/10.1371/journal.pone.0004998
Andolfo I, Liguori L, Antonellis PD et al (2012) The micro-RNA 199b–5p regulatory circuit involves Hes1, CD15, and epigenetic modifications in medulloblastoma. Neuro Oncol 14:596–612. https://doi.org/10.1093/neuonc/nos002
Antonellis PD, Medaglia C, Cusanelli E et al (2011) MiR-34a targeting of notch ligand delta-Like 1 impairs CD15 /CD133 tumor-propagating cells and supports neural differentiation in medulloblastoma. PLoS ONE. https://doi.org/10.1371/journal.pone.0024584
Hemmesi K, Squadrito ML, Mestdagh P et al (2015) miR-135aInhibits cancer stem cell-driven medulloblastoma development by directly repressingArhgef6expression. Stem Cells 33:1377–1389. https://doi.org/10.1002/stem.1958
Catanzaro G, Besharat ZM, Garg N et al (2016) MicroRNAs-proteomic networks characterizing human medulloblastoma-SLCs. Stem Cells Int 2016:1–10. https://doi.org/10.1155/2016/2683042
Kaid C, Silva PBG, Cortez BA et al (2015) miR-367 promotes proliferation and stem-like traits in medulloblastoma cells. Cancer Sci 106:1188–1195. https://doi.org/10.1111/cas.12733
Samaraweera L, Grandinetti KB, Huang R et al (2014) MicroRNAs define distinct human neuroblastoma cell phenotypes and regulate their differentiation and tumorigenicity. BMC Cancer. https://doi.org/10.1186/1471-2407-14-309
Foley NH, Bray I, Watters KM et al (2011) MicroRNAs 10a and 10b are potent inducers of neuroblastoma cell differentiation through targeting of nuclear receptor corepressor 2. Cell Death Differ 18:1089–1098. https://doi.org/10.1038/cdd.2010.172
Beveridge NJ, Tooney PA, Carroll AP et al (2009) Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cell Signal 21:1837–1845. https://doi.org/10.1016/j.cellsig.2009.07.019
Zhao Z, Ma X, Hsiao T-H et al (2014) A high-content morphological screen identifies novel microRNAs that regulate neuroblastoma cell differentiation. Oncotarget 5:2499–2512. https://doi.org/10.18632/oncotarget.1703
Das E, Bhattacharyya NP (2014) MicroRNA-432 contributes to dopamine cocktail and retinoic acid induced differentiation of human neuroblastoma cells by targeting NESTIN and RCOR1 genes. FEBS Lett 588:1706–1714. https://doi.org/10.1016/j.febslet.2014.03.015
Zhao Z, Ma X, Sung D et al (2015) microRNA-449a functions as a tumor suppressor in neuroblastoma through inducing cell differentiation and cell cycle arrest. RNA Biol 12:538–554. https://doi.org/10.1080/15476286.2015.1023495
Chen H, Shalom-Feuerstein R, Riley J et al (2010) miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development and embryonic stem cells differentiation, and control neurite outgrowth in vitro. Biochem Biophys Res Commun 394:921–927. https://doi.org/10.1016/j.bbrc.2010.03.076
Das S, Bryan K, Buckley PG et al (2012) Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene 32:2927–2936. https://doi.org/10.1038/onc.2012.311
Ren X, Bai X, Zhang X et al (2015) quantitative nuclear proteomics identifies that miR-137-mediated EZH2 Reduction Regulates Resveratrol-induced Apoptosis of Neuroblastoma cells*. Mol Cell Proteom 14:316–328. https://doi.org/10.1074/mcp.M114.041905
Molenaar JJ, Domingo-Fernández R, Ebus ME et al (2012) LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet 44:1199–1206. https://doi.org/10.1038/ng.2436
Chen J, Wang P, Cai R et al (2019) SLC34A2 promotes neuroblastoma cell stemness via enhancement of miR-25/Gsk3β-mediated activation of Wnt/β-catenin signaling. FEBS Open Bio 9:527–537. https://doi.org/10.1002/2211-5463.12594
Besharat ZM, Abballe L, Cicconardi F et al (2018) Foxm1 controls a pro-stemness microRNA network in neural stem cells. Sci Rep. https://doi.org/10.1038/s41598-018-21876-y
Huang S, Yang J-Y (2015) Targeting the hedgehog pathway in pediatric medulloblastoma. Cancers 7:2110–2123. https://doi.org/10.3390/cancers7040880
Wickström M, Dyberg C, Shimokawa T et al (2012) Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growthin vitroandin vivo. Int J Cancer 132:1516–1524. https://doi.org/10.1002/ijc.27820
Ames HM, Yuan M, Vizcaíno MA et al (2016) MicroRNA profiling of low-grade glial and glioneuronal tumors shows an independent role for cluster 14q32.31 member miR-487b. Mod Pathol 30:204–216. https://doi.org/10.1038/modpathol.2016.177
Jha P, Agrawal R, Pathak P et al (2015) Genome-wide small noncoding RNA profiling of pediatric high-grade gliomas reveals deregulation of several miRNAs, identifies downregulation of snoRNA cluster HBII-52 and delineates H3F3A and TP53 mutant-specific miRNAs and snoRNAs. Int J Cancer 137:2343–2353. https://doi.org/10.1002/ijc.29610
Lucon DR, Rocha CDS, Craveiro RB et al (2013) Downregulation of 14q32 microRNAs in primary human desmoplastic medulloblastoma. Front Oncol. https://doi.org/10.3389/fonc.2013.00254
Gattolliat C-H, Thomas L, Ciafrè SA et al (2011) Expression of miR-487b and miR-410 encoded by 14q32.31 locus is a prognostic marker in neuroblastoma. Br J Cancer 105:1352–1361. https://doi.org/10.1038/bjc.2011.388
Cheng Y, He C, Wang M et al (2019) Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. https://doi.org/10.1038/s41392-019-0095-0
Chang JC (2016) Cancer stem cells. Medicine. https://doi.org/10.1097/2FMD.0000000000004766
Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A (2017) Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol 44:10–24. https://doi.org/10.1016/j.semcancer.2017.02.011
Su JM, Li X-N, Thompson P et al (2010) Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a children’s oncology group report. Clin Cancer Res 17:589–597. https://doi.org/10.1158/1078-0432.CCR-10-0738
Su JMF, Murray JC, Mcnall-Knapp RY et al (2020) A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.28283
Masoudi A, Elopre M, Amini E, Nagel ME, Ater JL, Gopalakrishnan V, Wolff JE (2008) Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res 28:2437–2442
Tilburg CMV, Milde T, Witt R et al (2019) Phase I/II intra-patient dose escalation study of vorinostat in children with relapsed solid tumor, lymphoma, or leukemia. Clin Epigenetics. https://doi.org/10.1186/s13148-019-0775-1
Hummel TR, Wagner L, Ahern C et al (2013) A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a children’s oncology group phase 1 consortium study. Pediatr Blood Cancer 60:1452–1457. https://doi.org/10.1002/pbc.24541
Dubois SG, Groshen S, Park JR et al (2015) Phase I study of vorinostat as a radiation sensitizer with 131I-metaiodobenzylguanidine (131I-MIBG) for patients with relapsed or refractory neuroblastoma. Clin Cancer Res 21:2715–2721. https://doi.org/10.1158/1078-0432.CCR-14-3240
National Cancer Institute (NCI) (2016) A phase 1 study of entinostat, an oral histone deacetylase inhibitor, in pediatric patients with recurrent or refractory solid tumors, including CNS tumors and lymphoma. Identifier NCT02780804. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02780804
University Hospital Heidelberg (2019) Exploratory multinational phase I/II combination study of nivolumab and entinostat in children and adolescents with refractory high-risk malignancies. Identifier NCT03838042. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03838042
National Cancer Institute (NCI) (2010) A randomized phase II/III study of vorinostat and local irradiation OR temozolomide and local irradiation OR bevacizumab and local irradiation followed by maintenance bevacizumab and temozolomide in children with newly diagnosed high-grade gliomas. Identifier NCT01236560. Retrieved from https://clinicaltrials.gov/ct2/show/NCT01236560
Dana-Farber, Cancer Institute (2020) Phase 1 trial of marizomib alone and in combination with panobinostat for children with diffuse intrinsic pontine glioma. Identifier NCT04341311. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04341311
The Hospital for Sick Children (2017) Phase I/Ib trial of combined 5'azacitidine and carboplatin for recurrent/refractory pediatric brain and solid tumors. Identifier NCT03206021. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03206021
Ganesan A, Arimondo PB, Rots MG et al (2019) The timeline of epigenetic drug discovery: from reality to dreams. Clin Epigenetics. https://doi.org/10.1186/s13148-019-0776-0
Italiano A et al (2018) Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19:649–659. https://doi.org/10.1016/S1470-2045(18)30145-1
Janssen Research & Development, LLC (2018) A Phase 1, first-in-human, open-label study of the safety, pharmacokinetics, and pharmacodynamics of JNJ-64619178, an inhibitor of protein arginine methyltransferase 5 (PRMT5) in subjects with advanced cancers. Identifier NCT03573310. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03573310
Dartmouth-Hitchcock Medical Center (2013) Evaluating the expression levels of microRNA-10b in patients with gliomas. Identifier NCT01849952. Retrieved from https://clinicaltrials.gov/ct2/show/NCT01849952
Fouladi M et al (2010) Pediatric phase I trial and pharmacokinetic study of vorinostat: a children’s oncology group phase I consortium report. J Clin Oncol 28:3623–3629. https://doi.org/10.1200/JCO.2009.25.9119
Muscal JA et al (2013) A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a children’s oncology group phase I consortium study (ADVL0916). Pediatr Blood Cancer 60:390–395. https://doi.org/10.1002/pbc.24271
M.D. Anderson Cancer Center (2015) Vorinostat and temsirolimus with or without radiation therapy in treating younger patients with newly diagnosed or progressive diffuse intrinsic pontine glioma. Identifier NCT02420613. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02420613
New Approaches to Neuroblastoma Therapy Consortium (2017) MIBG with dinutuximab +/− vorinostat. Identifier NCT03332667. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03332667
Jubilant DraxImage Inc. (2018) A study of therapeutic iobenguane (131-I) and vorinostat for recurrent or progressive high-risk neuroblastoma subjects (OPTIMUM). Identifier NCT03561259. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03561259
Pinto N et al (2018) Phase I study of vorinostat in combination with isotretinoin in patients with refractory/recurrent neuroblastoma: a new approaches to neuroblastoma therapy (NANT) trial. Pediatr Blood Cancer 65:e27023. https://doi.org/10.1002/pbc.27023
New York Medical College (2020) Vorinostat in combination with chemotherapy in relapsed/refractory solid tumors and CNS malignancies (NYMC195). Identifier NCT04308330. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04308330
Leary SES et al (2022) Vorinostat and isotretinoin with chemotherapy in young children with embryonal brain tumors: a report from the pediatric brain tumor consortium (PBTC-026). Neuro Oncol 24:1178–1190. https://doi.org/10.1093/neuonc/noab293
Witt O et al (2012) Phase I/II intra-patient dose escalation study of vorinostat in children with relapsed solid tumor, lymphoma or leukemia. Klin Padiatr 224:398–403. https://doi.org/10.1186/s13148-019-0775-1
Peters KB et al (2018) Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol 137:349–356. https://doi.org/10.1007/s11060-017-2724-1
Su JM et al (2022) Phase I/II trial of vorinostat and radiation and maintenance vorinostat in children with diffuse intrinsic pontine glioma: a children’s oncology group report. Neuro Oncol 24:655–664. https://doi.org/10.1093/neuonc/noab188
Wake Forest University Health Sciences (2015) Pediatric precision laboratory advanced neuroblastoma therapy (PEDS-PLAN). Identifier NCT02559778. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02559778
DuBois SG et al (2021) Randomized phase II trial of MIBG versus MIBG, vincristine, and irinotecan versus MIBG and vorinostat for patients with relapsed or refractory neuroblastoma: a report from NANT consortium. J Clin Oncol 39:3506–3514. https://doi.org/10.1200/JCO.21.00703
Wolff JE et al (2008) Valproic acid was well tolerated in heavily pretreated pediatric patients with high-grade glioma. J Neurooncol 90:309–314. https://doi.org/10.1007/s11060-008-9662-x
Felix FH, Trompieri NM, de Araujo OL, da Trindade KM, Fontenele JB (2011) Potential role for valproate in the treatment of high–risk brain tumors of childhood-results from a retrospective observational cohort study. Pediatr Hematol Oncol 28:556–570. https://doi.org/10.3109/08880018.2011.563774
Wolff JE et al (2012) Treatment of recurrent diffuse intrinsic pontine glioma: the MD anderson cancer center experience. J Neurooncol 106:391–397. https://doi.org/10.1007/s11060-011-0677-3
Su JM et al (2020) A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr Blood Cancer 67:e28283. https://doi.org/10.1002/pbc.28283
University of California, San Francisco (2021) Combination therapy for the treatment of diffuse midline gliomas. Identifier NCT05009992. Retrieved from https://clinicaltrials.gov/ct2/show/NCT05009992
University of Göttingen (2017) International Cooperative Phase III Trial of the HIT-HGG Study Group (HIT-HGG-2013) (HIT-HGG-2013). Identifier NCT03243461. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03243461
Bukowinski A et al (2021) A phase 1 study of entinostat in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: trial ADVL1513, pediatric early phase-clinical trial network (PEP-CTN). Pediatr Blood Cancer 68:e28892. https://doi.org/10.1002/pbc.28892
van Tilburg CM et al (2020) INFORM2 NivEnt: the first trial of the INFORM2 biomarker driven phase I/II trial series: the combination of nivolumab and entinostat in children and adolescents with refractory high-risk malignancies. BMC Cancer 20:523. https://doi.org/10.1186/s12885-020-07008-8
Pediatric Brain Tumor Consortium (2016) Trial of panobinostat in children with diffuse intrinsic pontine glioma (PBTC-047). Identifier NCT02717455. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02717455
The University of Texas Health Science Center, Houston (2020) infusion of panobinostat (MTX110) into the fourth ventricle in children and adults with recurrent medulloblastoma. Identifier NCT04315064. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04315064
Cheng-Chia (Fred) Wu (2021) Non-invasive focused ultrasound (FUS) with oral panobinostat in children with progressive diffuse midline glioma (DMG). Identifier NCT04804709. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04804709
Luca Szalontay (2020) CED of MTX110 newly diagnosed diffuse midline gliomas. Identifier NCT04264143. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04264143
National Cancer Institute (NCI) (2017) Targeted therapy directed by genetic testing in treating pediatric patients with relapsed or refractory advanced solid tumors, non-hodgkin lymphomas, or histiocytic disorders (The pediatric MATCH screening trial). Identifier NCT03155620. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03155620
National Cancer Institute (NCI) (2017) Tazemetostat in treating patients with relapsed or refractory advanced solid tumors, non-hodgkin lymphoma, or histiocytic disorders With EZH2, SMARCB1, or SMARCA4 gene mutations (a pediatric MATCH treatment trial). Identifier NCT03213665. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03213665
George RE et al (2010) Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a children’s oncology group study. Pediatr Blood Cancer 55:629–638. https://doi.org/10.1002/pbc.22607
Krishnadas DK et al (2015) A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 64:1251–1260. https://doi.org/10.1007/s00262-015-1731-3
AbbVie (2017) A Study of the Safety and Pharmacokinetics of Venetoclax in Pediatric and Young Adult Patients With Relapsed or Refractory Malignancies. Identifier NCT03236857. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03236857
Fenaux P et al (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10:223–232. https://doi.org/10.1016/S1470-2045(09)70003-8
Kantarjian H et al (2006) Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106:1794–1803. https://doi.org/10.1002/cncr.21792
Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R (2007) FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 12:1247–1252. https://doi.org/10.1634/theoncologist.12-10-1247
Piekarz RL et al (2009) Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 27:5410–5417. https://doi.org/10.1200/JCO.2008.21.6150
O’Connor OA et al (2015) Belinostat in patients with relapsed or refractory peripheral T-Cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol 33:2492–2499. https://doi.org/10.1200/JCO.2014.59.2782
Laubach JP et al (2021) Efficacy and safety of oral panobinostat plus subcutaneous bortezomib and oral dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma (PANORAMA 3): an open-label, randomised, phase 2 study. Lancet Oncol 22:142–154. https://doi.org/10.1016/S1470-2045(20)30680-X
Santos FP, Kantarjian H, Garcia-Manero G, Issa JP, Ravandi F (2010) Decitabine in the treatment of myelodysplastic syndromes. Expert Rev Anticancer Ther 10:9–22. https://doi.org/10.1586/era.09.164
Götze K et al (2010) Azacitidine for treatment of patients with myelodysplastic syndromes (MDS): practical recommendations of the German MDS study group. Ann Hematol 89:841–850. https://doi.org/10.1007/s00277-010-1015-0
Duvic M et al (2009) Evaluation of the long-term tolerability and clinical benefit of vorinostat in patients with advanced cutaneous T-cell lymphoma. Clin Lymphoma Myeloma 9:412–416. https://doi.org/10.3816/CLM.2009.n.082
Acknowledgements
The authors would like to thank Dra. Marialva Sinigaglia her images expertise.
Funding
This article was supported by Ministry of Health/CNPq/FAPERGS PPSUS (Grant Number 21/2551–0000114-3 to L.G), the Children’s Cancer Institute (ICI), the National Council for Scientific and Technological Development (CNPq, MCTI, Brazil; grant 305647/2019-9 to R.R. and scholarship to N.H.F.), and the William Donald Nash Brain Tumour Research Fellowship, Brain Tumour Foundation of Canada (Canada; C.N.); the Swifty Foundation (Canada; C.N.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. Literature search and data analysis were performed by NHF and MCJ. The first draft of the manuscript was written by NHF and all authors revised and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Disclosure of potential conflicts of interest: The authors declare no competing interests.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this article.
Research involving human participants and animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Freire, N.H., Jaeger, M.d., de Farias, C.B. et al. Targeting the epigenome of cancer stem cells in pediatric nervous system tumors. Mol Cell Biochem 478, 2241–2255 (2023). https://doi.org/10.1007/s11010-022-04655-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04655-2