Abstract
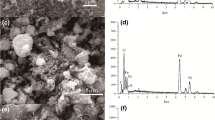
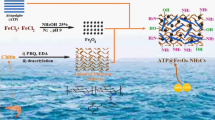
Using the waste basalt powder, ferric chloride (FeCl3·6H2O), ferrous sulfate (FeSO4·7H2O), 3-aminotrimethoxysilane (3-TMSPT) and Chitosan (CTS) as raw materials, through hydrothermal synthesis, in-situ synthesis, cross-linking and grafting methods, the amino-modified chitosan/magnetic zeolite composite (NH2-CTS/MZ) was prepared. The samples were characterized by SEM, XRD, VSM, BET, FTIR and XPS. The removal ability and adsorption mechanism of U(VI) in an aqueous solution by NH2-CTS/MZ were systematically studied. The results showed that under the conditions of 308 K and pH = 5, NH2-CTS/MZ reached adsorption equilibrium within 180 min, and the equilibrium adsorption capacity was 749.5 mg·g−1. The adsorption isotherm conforms to the Langmuir isotherm model (R2 = 0.998), and the adsorption kinetics conforms to the quasi-secondary kinetic model (R2 = 0.987). The adsorption behaviour is a spontaneous endothermic process. This study shows that NH2-CTS/MZ has a good application prospect in the removal of uranyl ions.














Similar content being viewed by others
References
Jinhua W, Mingjia Y, Sihao L, Weizhao Y, Huaitian B, Li L, Ping L, Hong D, Xiangyu Z (2021) Preparation of highly dispersive and antioxidative nano zero-valent iron for the removal of hexavalent chromium. Chemosphere 262:127733–127741
Chen C, Zhang X, Jiang T, Li M, Peng Y, Liu X, Ye J, Hua Y (2021) Removal of uranium(VI) from aqueous solution by Mg(OH)2-coated nanoscale zero-valent iron: Reactivity and mechanism. J Environ Chem Eng 9(1):104706–104734
Wang B, Deng C, Ma W, Sun Y (2021) Modified nanoscale zero-valent iron in persulfate activation for organic pollution remediation: a review. Environ Sci Pollut Res 28(26):34229–34247
Yin N, Ai Y, Xu Y, Ouyang Y, Yang P (2020) Preparation of magnetic biomass-carbon aerogel and its application for adsorption of uranium(VI). J Radioanal Nucl Chem 326(2):1307–1321
Suzuki T, Kawasaki T, Takao K, Harada M, Nogami M, Ikeda Y (2012) A study on selective precipitation ability of cyclic urea to U(VI) for developing reprocessing system based on precipitation method. J Nucl Sci Technol 49(10):1010–1017
Xu YH, Ke GJ, Yin J, Lei WR, Yang PF (2019) Synthesis of thiol-functionalized hydrotalcite and its application for adsorption of uranium (VI). J Radioanal Nucl Chem 319(3):791–803
Yin L, Hu B, Zhuang L, Fu D, Li J, Hayat T, Alsaedi A, Wang X (2020) Synthesis of flexible cross-linked cryptomelane-type manganese oxide nanowire membranes and their application for U(VI) and Eu(III) elimination from solutions. Chem Eng J 381:122744–122753
Dan H, Zhongyu R, Xiaoyu L, Qi J (2021) Mechanism of stability and transport of chitosan-stabilized nano zero-valent iron in saturated porous media. Int J Environ Res Public Health 18(10):5115–5115
Yang PF, Xu YX, Yin N, Ai Y (2020) Preparation of uniform highly dispersed Mg-Al-LDHs and their adsorption performance for chloride ions. Ind Eng Chem Res 59(22):10697–10704
Rakesh S, Rao. (2015) Separation of U(VI) and Th(IV) from Nd(III) by cross-current solvent extraction mode using Tri- iso -amyl phosphate as the extractant. Solvent Extr Ion Exch 33(5):448–461
Sahu A, Vincent T, Shah JG, Wattal PK (2014) Catalytic reduction of U(VI) to U(IV) using hydrogen with platinum loaded on alumina and silica. J Radioanal Nucl Chem 300(1):163–167
Yujun C, Haoran D, Tianwei H (2021) CaCO3 coated nanoscale zero-valent iron (nZVI) for the removal of chromium(VI) in aqueous solution. Sep Purif Technol 257:117967–117969
Cheira MF, Mira HI, Sakr AK, Mohamed SA (2019) Adsorption of U(VI) from acid solution on a low-cost sorbent: equilibrium, kinetic, and thermodynamic assessments. Nucl Sci Tech 30(10):1–18
Zhang H, Liu Z, Hu P, Liu T, Wu W (2013) Adsorption of U(VI) onto kaolin studied by batch method. J Radioanal Nucl Chem 298(1):611–619
Şenol ZM, Şimşek S, Özer A, Şenol Arslan D (2020) Synthesis and characterization of chitosan–vermiculite composite beads for removal of uranyl ions: isotherm, kinetics and thermodynamics studies. J Radioanal Nucl Chem 327(1):159–173
Bayramoglu G, Arica MY (2019) Star type polymer grafted and polyamidoxime modified silica coated-magnetic particles for adsorption of U(VI) ions from solution. Chem Eng Res Des 147:146–159
Şenol ZM, Şimşek S, Ulusoy Hİ, Özer A (2020) Synthesis and characterization of a polyacrylamide-dolomite based new composite material for efficient removal of uranyl ions. J Radioanal Nucl Chem 324(1):317–330
Arica TA, Ayas E, Arica MY (2017) Magnetic MCM-41 silica particles grafted with poly(glycidylmethacrylate) brush: modification and application for removal of direct dyes. Microporous Mesoporous Mater 243:164–175
Bayramoglu G, Arica MY (2017) Polyethylenimine and tris(2-aminoethyl)amine modified p(GA–EGMA) microbeads for sorption of uranium ions: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 312:293–303
Bayramoglu G, Yakup Arica M (2015) Amidoxime functionalized Trametes trogii pellets for removal of uranium(VI) from aqueous medium. J Radioanal Nucl Chem 307:373–384
H, E. N., S, A. R. A., & M, M. (2021) Amidoxime modified chitosan based ion-imprinted polymer for selective removal of uranyl ions. Carbohyd Polym 256:117509–117515
F, H. M., Amr, F., Z, E. K., Yuezhou, W., Eric, G., & A, H. N. (2021) Phosphorylation of Guar gum/magnetite/chitosan nanocomposites for Uranium (VI) sorption and antibacterial applications. Molecules (Basel, Switzerland) 26(7):1920–1920
Wang X, Shao D, Hou G, Wang X, Alsaedi A, Ahmad B (2015) Uptake of Pb(II) and U(VI) ions from aqueous solutions by the ZSM-5 zeolite. J Mol Liq 207:338–342
Abdolmohammad-Zadeh H, Ayazi Z, Naghdi Z (2019) Nickel oxide/chitosan nano-composite as a magnetic adsorbent for pre-concentration of Zn(II) ions. J Magn Magn Mater 488:165311–165320
Jiang X, Wang H, Hu E, Lei Z, Fan B, Wang Q (2020) Efficient adsorption of uranium from aqueous solutions by microalgae based aerogel. Microp Mesop Mater 305(C):110383–110408
Yeqi W (2020) Study on the adsorption properties of modified magnetic chitosan beads for U(VI)[D]. North China Electric Power University (Beijing), 40–41
Liao Y, Wang M, Chen DJ (2018) Production of three-dimen-sional porous polydopamine-functionalized attapulgite/chitosan aerogel for uranium(VI) adsorption. J Radioanal Nucl Chem 316(2):635–647
Esposito S, Dell’Agli G, Marocco A, Bonelli B, Allia P, Tiberto P, Barrera G, Manzoli M, Arletti R, Pansini M (2018) Magnetic metal-ceramic nanocomposites obtained from cation-exchanged zeolite by heat treatment in reducing atmosphere. Microp Mesop Mater 268:131–143
Ramos-Guivar JA, Taipe K, Schettino MA et al (2020) Improved removal capacity and equilibrium time of maghemite nanoparticles growth in Zeolite Type 5A for Pb(II) adsorption[J]. Nanomaterials 10(9):1668–1687
Saghatchi H, Ansari R, Mousavi HZ (2018) Highly efficient adsorptive removal of uranyl ions from aqueous solutions using dicalcium phosphate nanoparticles as a superabsorbent. Nucl Eng Technol 50(7):1112–1119
Dawood S, Sen TK (2012) Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res 46(6):1933–1946
Arica MY, Bayramoglu G (2016) Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chem 310(2):711–724
Purnomo CW, Salim C, Hinode H (2012) Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous Mesoporous Mater 162:6–13
Jamil TS, Ghafar HHA, Ibrahim HS, El-Maksoud IHA (2011) Removal of methylene blue by two zeolites prepared from naturally occurring Egyptian kaolin as cost effective technique. Solid State Sci 13(10):1844–1851
Wang S, Wang L, Li Z, Zhang P, Shi W (2021) Highly efficient adsorption and immobilization of U(VI) from aqueous solution by alkalized MXene-supported nanoscale zero-valent iron. J Hazard Mater 408:124949–124956
Şenol ZM, Şenol Arslan D, Şimşek S (2019) Preparation and characterization of a novel diatomite-based composite and investigation of its adsorption properties for uranyl ions. J Radioanal Nucl Chem 321(3):791–803
Simsek S, Senol ZM, Ulusoy HI (2017) Synthesis and characterization of a composite polymeric material including chelating agent for adsorption of uranyl ions. J Hazard Mater 338:437–446
Hamza MF, Roux J-C, Guibal E (2018) Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem Eng J 344:124–137
Pang H, Diao Z, Wang X, Ma Y, Yu S, Zhu H, Chen Z, Hu B, Chen J, Wang X (2019) Adsorptive and reductive removal of U(VI) by Dictyophora indusiate -derived biochar supported sulfide NZVI from wastewater. Chem Eng J 366:368–377
Bai J, Chu J, Yin X, Wang J, Tian W, Huang Q, Jia Z, Wu X, Guo H, Qin Z (2020) Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem Eng J 391:123553–123590
Acknowledgements
This study was financially supported by the Natural Science Foundation of Hunan Province (2021JJ30568)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, M., Ai, Y., Zhao, L. et al. Preparation of NH2-CTS/MZ composites and their adsorption behavior and mechanism on uranium ions. J Radioanal Nucl Chem 330, 963–978 (2021). https://doi.org/10.1007/s10967-021-07991-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07991-7