Abstract
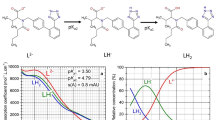
Bedaquiline (trade name Sirturo) is an antibiotic used to treat pulmonary tuberculosis that is resistant to other antibiotics. The pH-spectrophotometric and pH-potentiometric titrations allowed the measurement of two near successive and one distant dissociation constants. The neutral bedaquiline LH molecule was able to protonate and dissociate in pure water to form soluble species L−, LH, \({\text{LH}}_{2}^{ + }\), \({\text{LH}}_{3}^{2 + }\) and \({\text{LH}}_{4}^{3 + }\). In the pH range 2–7, three dissociation constants can be reliably estimated. REACTLAB (UV-metric spectral analysis) values are: \({\text{p}}K_{{{\text{a}}1}}^{T}\) = 3.91(09), \({\text{p}}K_{{{\text{a}}2}}^{T}\) = 4.58(12) and \({\text{p}}K_{{{\text{a3}}}}^{T}\) = 5.26(07) at 25 °C and \({\text{p}}K_{{{\text{a}}1}}^{T}\) = 3.61(30), \({\text{p}}K_{{{\text{a2}}}}^{T}\) = 4.44(15) and \({\text{p}}K_{{{\text{a3}}}}^{T}\) = 5.54(33) at 37 °C. ESAB (pH-metric analysis) values are: \({\text{p}}K_{{{\text{a}}1}}^{T}\) = 3.21(39), \({\text{p}}K_{{{\text{a2}}}}^{T}\) = 3.68(31) and \({\text{p}}K_{{{\text{a3}}}}^{T}\) = 5.21(42) at 25 °C and \({\text{p}}K_{{{\text{a}}1}}^{T}\) = 3.31(12), \({\text{p}}K_{{{\text{a2}}}}^{T}\) = 3.67(15) and \({\text{p}}K_{{{\text{a3}}}}^{T}\) = 5.73(08) at 37 °C. Molar enthalpy ΔH0, molar entropy ΔS0 and Gibbs energy ΔG0 were calculated from the spectra using the dependence of ln K on 1/T. The potentiometric data showed positive enthalpy ΔH0(pKa1) = 85.49 kJ·mol−1, ΔH0(pKa2) = 86.42 kJ·mol−1, and ΔH0(pKa3) = 65.84 kJ·mol−1 values and the dissociation reactions were endothermic. The entropy ΔS0 at 25 °C was positive for the three dissociation constants ΔS0(pKa1) = 217.47 J·K−1·mol−1, ΔS0(pKa2) = 204.87 J·K−1·mol−1, and ΔS0(pKa3) = 92.63 J·K−1·mol−1 at 25 °C and proved irreversible dissociation reactions.
Graphic Abstract









Similar content being viewed by others
References
Wikipedia: Bedaquiline. Wikipedia-https://pubchem.ncbi.nlm.nih.gov/compound/Bedaquiline (2013)
Multum, C.: Bedaquiline. Drugs.com https://www.drugs.com/mtm/Bedaquiline.html (2020)
Prakash, S.: Pharmacovigilance in India. Indian J. Pharmacol. 39(3), 1 (2007). https://doi.org/10.4103/0253-7613.33430
Sarathy, J.P., Gruber, G., Dick, T.: Re-Understanding the mechanisms of action of the anti-mycobacterial drug bedaquiline. Antibiotics 8(4), 261–273 (2019). https://doi.org/10.3390/antibiotics8040261
Koul, A., Dendouga, N., Vergauwen, K., Molenberghs, B., Vranckx, L., Willebrords, R., Ristic, Z., Lill, H., Dorange, I., Guillemont, J., Bald, D., Andries, K.: Diarylquinolines target subunit C of mycobacterial ATP synthase. Nat. Chem. Biol. 3(6), 323–324 (2007). https://doi.org/10.1038/nchembio884
Segala, E., Sougakoff, W., Nevejans-Chauffour, A., Jarlier, V., Petrella, S.: New mutations in the mycobacterial ATP synthase: new insights into the minding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob. Agents Chemother. 56(5), 2326–2334 (2012). https://doi.org/10.1128/AAC.06154-11
Goldberg, R.N., Kishore, N., Lennen, R.M.: Thermodynamic quantities for the ionization reactions of buffers. J. Phys. Chem. Ref. Data 31(2), 231–370 (2002). https://doi.org/10.1063/1.1416902
Williams, H.D., Trevaskis, N.L., Charman, S.A., Shankere, R.M., Charman, W.N., Pouton, C.E., Porter, C.J.H.: Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 65(1), 315–499 (2013). https://doi.org/10.1124/pr.112.005660
Al-Bedair, L.A.: Potentiometric studies on the binary and mixed ligand complexes in solution: Zn(II)– amlodipine–amino acids systems. Orient. J. Chem. 32(1), 609–615 (2016). https://doi.org/10.13005/Ojc/320169
Alderighi, L., Gans, P., Lenco, A., Peters, D., Sabatini, A., Vacca, A.: Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 184, 311–318 (1999). https://doi.org/10.1016/S0010-8545(98)00260-4
Byrne, L.A., Hynes, M.J., Connolly, C.D., Murphy, R.A.: Analytical determination of apparent stability constants using a copper ion selective electrode. J. Inorg. Biochem. 105(12), 1656–1661 (2011). https://doi.org/10.1016/j.jinorgbio.2011.07.016
De Stefano, C., Princi, P., Rigano, C., Sammartano, S.: Computer analysis of equilibrium data in solution ESAB2M: an improved version of the ESAB program. Ann. Chim. 77(7–8), 643–675 (1987)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43(10), 1739–1753 (1996). https://doi.org/10.1016/0039-9140(96)01958-3
Gans, P., Sabatini, A., Vacca, A.: Hyperquad computer-program suite. Abstr. Pap. Am. Chem. Soc. 219, U763–U763 (2000)
Gans, P., Sabatini, A., Vacca, A.: Simultaneous calculation of equilibrium constants and standard formation enthalpies from calorimetric data for systems with multiple equilibria in solution. J. Solution Chem. 37(4), 467–476 (2008). https://doi.org/10.1007/s10953-008-9246-6
Allen, R.I., Box, K.J., Comer, J.E.A., Peake, C., Tam, K.Y.: Multiwavelength spectrophotometric determination of acid dissociation constants of ionizable drugs. J. Pharm. Biomed. Anal. 17(4–5), 699–712 (1998). https://doi.org/10.1016/S0731-7085(98)00010-7
Meloun, M., Ferenčíková, Z., Niesnerová, I., Pekárek, T.: Oligomers-model building in protonation equilibria of sitagliptin. Cent. Eur. J. Chem. 11(11), 1799–1807 (2013). https://doi.org/10.2478/s11532-013-0304-6
Krotz-Vogel, W., Hoppe, H.C.: The PALLAS parallel programming environment. Lect. Notes Comput. Sci. 1332, 257–266 (1997). https://doi.org/10.1007/3-540-63697-8_93
Krotz-Vogel, W., Hoppe, H.C.: PALLAS parallel tools—a uniform programming environment from workstations to teraflop computers. Adv. Parallel Comput. 12, 349–358 (1998)
Meloun, M., Syrový, T., Bordovská, S., Vrána, A.: Reliability and uncertainty in the estimation of pK(a) by least squares nonlinear regression analysis of multiwavelength spectrophotometric pH titration data. Anal. Bioanal. Chem. 387(3), 941–955 (2007). https://doi.org/10.1007/s00216-006-0993-1
Japertas, P., Lanevskij, K., Sazonovas, A.: ACD/Percepta structure design engine: virtual enumeration and screening of physchem properties for 10(16) compounds in real time. Abstr. Pap. Am. Chem. Soc. 248 (2014)
ACD/pKa DB. In: pK Prediction Software. Advanced Chemistry Development, Inc., Toronto, Canada (2021). https://www.acdlabs.com
ACD/Labs pKa Predictor 3.0. In: Inc., A.C.D. (ed.) Toronto, Canada (2007). https://www.acdlabs.com, 2021
Balogh, G.T., Gyarmati, B., Nagy, B., Molnar, L., Keseru, G.M.: Comparative evaluation of in silico pK(a) prediction tools on the gold standard dataset. Qsar Comb. Sci. 28(10), 1148–1155 (2009). https://doi.org/10.1002/gsar.200960036
ChemAxon: MARVINSketch 4.1.8. In: ChemAxon, 2007. MarvinSketch 4.1.8. XhemAxon Kft. Budapest, Hungary. http://www.chemaxon.com/products.html, vol. 2007. ChemAxon, 2007 XhemAxon Kft. Budapest, Hungary (2007)
Meloun, M., Bordovská, S., Syrový, T.: A novel computational strategy for the pK(a) estimation of drugs by non-linear regression of multiwavelength spectrophotometric pH-titration data exhibiting small spectral changes. J. Phys. Org. Chem. 20(9), 690–701 (2007). https://doi.org/10.1002/poc.1235
Manchester, J., Walkup, G., Rivin, O., You, Z.P.: Evaluation of pK(a) estimation methods on 211 drug like compounds. J. Chem. Inf. Model. 50(4), 565–571 (2010). https://doi.org/10.1021/ci100019p
Meloun, M., Bordovská, S.: Benchmarking and validating algorithms that estimate pK(a) values of drugs based on their molecular structures. Anal. Bioanal. Chem. 389(4), 1267–1281 (2007). https://doi.org/10.1007/s00216-007-1502-x
Meloun, M., Bordovská, S., Syrový, T., Vrána, A.: Tutorial on a chemical model building by least-squares non-linear regression of multiwavelength spectrophotometric pH-titration data. Anal. Chim. Acta 580(1), 107–121 (2006). https://doi.org/10.1016/j.aca.2006.07.043
Meloun, M., Čápová, A., Pilařová, L., Pekárek, T.: Multiwavelength UV-metric and pH-metric determination of the multiple dissociation constants of the Lesinurad. J. Pharm. Biomed. Anal. 158, 236–246 (2018). https://doi.org/10.1016/j.jpba.2018.05.047
Meloun, M., Havel, J., Högfeldt, E.: Computation of Solution Equilibria: A Guide to Methods in Potentiometry, Extraction, and Spectrophotometry. Ellis Horwood Series in Analytical Chemistry. Ellis Horwood, Chichester (1988)
Rodante, F., Fantauzzi, F.: Thermodynamic analysis of the ionization of ortho and para toluic acids—influence of the medium on the hyperconjugative and steric effects. Thermochim. Acta 109(2), 353–365 (1987). https://doi.org/10.1016/040-6031(87)80031-X
Meloun, M., Ferenčíková, Z.: Enthalpy–entropy compensation for some drugs dissociation in aqueous solutions. Fluid Phase Equil. 328, 31–41 (2012). https://doi.org/10.1016/j.fluid.2012.05.011
Debnath, A.K., Shusterman, A.J., Decompadre, R.L.L., Hansch, C.: The importance of the hydrophobic interaction in the mutagenicity of organic-compounds. Mutat. Res. 305(1), 63–72 (1994). https://doi.org/10.1016/0027-5107(94)90126-0
Gruber, C., Buss, V.: Quantum mechanically calculated properties for the development of quantitative structure–activity–relationships (Qsars)—pKa values of phenols and aromatic and aliphatic carboxylic-acids. Chemosphere 19(10–11), 1595–1609 (1989). https://doi.org/10.1016/0045-6535(89)90503-1
Shusterman, A.J.: Predicting mutagenicity—use a technique thats been useful in predicting efficacy. ChemTech 21(10), 624–627 (1991)
Trapani, G., Carotti, A., Franco, M., Latrofa, A., Genchi, G., Liso, G.: Structure affinity relationships of some alkoxycarbonyl-2h-pyrimido[2,1-B]benzothiazol-2-ones or alkoxycarbonyl-4h-pyrimido[2,1-B]benzothiazol-4-ones benzodiazepine receptor ligands. Eur. J. Med. Chem. 28(1), 13–21 (1993). https://doi.org/10.1016/0223-5234(93)90074-O
Aguerre, R.J., Suarez, C., Viollaz, P.E.: Enthalpy–entropy compensation in sorption phenomena—application to the prediction of the effect of temperature on food isotherms. J. Food Sci. 51(6), 1547–1549 (1986). https://doi.org/10.1111/j.1365-2621.1986.tb13856.x
Gabas, A.L., Telis-Romero, J., Menegalli, F.C.: Thermodynamic models for water sorption by grape skin and pulp. Dry. Technol. 17(4–5), 961–974 (1999). https://doi.org/10.1080/07373939908917584
Labuza, T.P.: Enthalpy–entropy compensation in food reactions. Food Technol-Chicago 34(2), 67–77 (1980)
Liu, L., Guo, Q.X.: Isokinetic relationship, isoequilibrium relationship, and enthalpy–entropy compensation. Chem. Rev. 101(3), 673–695 (2001). https://doi.org/10.1021/cr990416z
Meloun, M., Nečasová, V., Javůrek, M., Pekárek, T.: The dissociation constants of the cytostatic bosutinib by nonlinear least-squares regression of multiwavelength spectrophotometric and potentiometric pH-titration data. J. Pharm. Biomed. Anal. 120, 158–167 (2016). https://doi.org/10.1016/j.jpba.2015.12.012
Meloun, M., Pilařová, L., Čápová, A., Pekárek, T.: The overlapping thermodynamic dissociation constants of the antidepressant vortioxetine using UV-vis multiwavelength pH-titration data. J. Solution Chem. 47(5), 806–826 (2018). https://doi.org/10.1007/s10953-018-0757-5
Meloun, M., Říha, V., Žáček, J.: Piston microburette for dosing aggressive liquids. Chem. Listy 82(7), 765–767 (1988)
Leggett, D.J., McBryde, W.A.E.: General computer program for the computation of stability constants from absorbance data. Anal. Chem. 47(7), 1065–1070 (1975). https://doi.org/10.1021/ac60357a046
Maeder, M., King, P.: Analysis of chemical processes, determination of the reaction mechanism and fitting of equilibrium and/or rate constants. In: Varmuza, K. (ed.) Chemometrics in Practical Applications, pp.41–62. Rjeka, InTech
Meloun, M., Čapek, J., Mikšík, P., Brereton, R.G.: Critical comparison of methods predicting the number of components in spectroscopic data. Anal. Chim. Acta 423(1), 51–68 (2000). https://doi.org/10.1016/S0003-2670(00)01100-4
ORIGIN. In. OriginLab Corporation, One Roundhouse Plaza, Suite 303, Northampton, MA 01060, USA
Meloun, M., Militký, J.: Statistical Data Analysis: A Practical Guide, Complete with 1250 exercises and answer key on CD, 1st edn. Woodhead Publishing Limited, 80 High street Sawstone Cambridge, CB22 3HJ, UK, New Delhi, Cambridge, Oxford, Philadelphia (2011)
Meloun, M., Militký, J., Forina, M.: Chemometrics for Analytical Chemistry. PC-Aided Regression and Related Methods, vol. 2. Ellis Horwood, Chichester (1994), ISBN 0-13-123788-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.








Rights and permissions
About this article
Cite this article
Meloun, M., Cyrmonová, D., Javůrek, M. et al. Acid Dissociation Constants, Enthalpy, Entropy and Gibbs Energy of Bedaquiline by UV-Metric Spectral and pH-Metric Analysis. J Solution Chem 50, 315–339 (2021). https://doi.org/10.1007/s10953-021-01055-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-021-01055-w