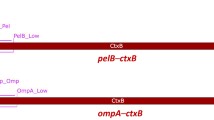
Abstract
Angiotensin-converting enzyme 2 (ACE2) has a specific interaction with the coronavirus spike protein, enabling its entry into human cells. This membrane enzyme converts angiotensin II into angiotensin 1–7, which has an essential role in protecting the heart and improving lung function. Many therapeutic properties have been attributed to the human recombinant ACE2 (hrACE2), especially in combating complications related to diabetes mellitus and hypertension, as well as, preventing the coronavirus from entering the target tissues. In the current study, we designed an appropriate gene construct for the hybrid protein containing the ACE2 catalytic subunit and the B subunit of cholera toxin (CTB-ACE2). This structural feature will probably help the recombinant hybrid protein enter the mucosal tissues, including the lung tissue. Optimization of this hybrid protein expression was investigated in BL21 bacterial host cells. Also, the hybrid protein was identified with an appropriate antibody using the ELISA method. A large amount of the hybrid protein (molecular weight of ~ 100 kDa) was expressed as the inclusion body when the induction was performed in the presence of 0.25 mM IPTG and 1% sucrose for 10 h. Finally, the protein structural features were assessed using several biophysical methods. The fluorescence emission intensity and oligomeric size distribution of the CTB-ACE2 suggested a temperature-dependent alteration. The β-sheet and α-helix were also dominant in the hybrid protein structure, and this protein also displays acceptable chemical stability. In overall, according to our results, the efficient expression and successful purification of the CTB-ACE2 protein may pave the path for its therapeutic applications against diseases such as covid-19, diabetes mellitus and hypertension.
Graphical Abstract














Similar content being viewed by others
References
Atlas SA (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm JMCP 13:9–20. https://doi.org/10.18553/jmcp.2007.13.s8-b.9
Verma A, Xu K, Du T, Zhu P, Liang Z, Liao S, Zhang J, Raizada MK, Grant MB, Li Q (2019) Expression of human ACE2 in Lactobacillus and Beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev 14:161–170. https://doi.org/10.1016/j.omtm.2019.06.007
Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637. https://doi.org/10.1002/path.1570
Lippi G, Lavie CJ, Henry BM, Sanchis-Gomar F (2020) Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin Chem Lab Med 58(2020):1415–1422. https://doi.org/10.1515/cclm-2020-0727
Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ (2005) Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280:30113–30119. https://doi.org/10.1074/jbc.M505111200
Aragão DS, Cunha TS, Arita DY, Andrade MCC, Fernandes AB, Watanabe IKM, Mortara RA, Casarini DE (2011) Purification and characterization of angiotensin converting enzyme 2 (ACE2) from murine model of mesangial cell in culture. Int J Biol Macromol 49:79–84. https://doi.org/10.1016/j.ijbiomac.2011.03.018
Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238–33243. https://doi.org/10.1074/jbc.M002615200
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417:822–828. https://doi.org/10.1038/nature00786
Xiao H, Nie X-T, Ji X-X, Yan S, Zhu B, Zhang Y-S (2020) Establishing prokaryotic expression system of angiotensin-converting enzyme 2 (ACE2) gene in pigs. BioRxiv. https://doi.org/10.1101/2020.03.12.988634
Ghahramani M, Yousefi R, Niazi A, Kurganov B (2020) The congenital cataract-causing mutations P20R and A171T are associated with important changes in the amyloidogenic feature, structure and chaperone-like activity of human αB-crystallin. Biopolymers 111:e23350
Teale FWJ, Weber G (1957) Ultraviolet fluorescence of the aromatic amino acids. Biochem J 65:476
Zhang Y-Z, Zhou B, Liu Y-X, Zhou C-X, Ding X-L, Liu Y (2008) Fluorescence study on the interaction of bovine serum albumin with p-aminoazobenzene. J Fluoresc 18:109–118
Khoshaman K, Oryan A, Moosavi-Movahedi AA, Tamaddon AM, Kurganov BI, Yousefi R, Abolmaali SS (2017) impact of different mutations at Arg54 on structure, chaperone-like activity and oligomerization state of human αA-crystallin: The pathomechanism underlying congenital cataract-causing mutations R54L, R54P and R54C. BBA-Proteins Proteom 1865:604–618
Maltas E (2014) Binding interactions of niclosamide with serum proteins. J Food Drug Anal 22:549–555
Sharma KK, Kumar GS, Murphy AS, Kester K (1998) Identification of 1, 1′-bi (4-anilino) naphthalene-5, 5′-disulfonic acid binding sequences in α-crystallin. J Biol Chem 273:15474–15478
Bortolotti A, Wong YH, Korsholm SS, Bahring NHB, Bobone S, Tayyab S, van de Weert M, Stella L (2016) On the purported “backbone fluorescence” in protein three-dimensional fluorescence spectra. Rsc Adv 6:112870–112876
Grigoryan KR, Shilajyan HA (2017) Fluorescence 2D and 3D spectra analysis of tryptophan, tyrosine and phenylalanine. Chem Biol 51:3–7
Whitmore L, Wallace BA (2004) DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res 32:W668–W673
Sadat A, Joye IJ (2020) Peak fitting applied to Fourier transform infrared and Raman spectroscopic analysis of proteins. Appl Sci 10:5918
Khaleghinejad SH, Shahsavani MB, Ghahramani M, Yousefi R (2023) Investigating the role of double mutations R12C/P20R, and R12C/R69C on structure, chaperone-like activity, and amyloidogenic properties of human αB-crystallin. Int J Biol Macromol 242:124590
Zhou C, Qi W, Lewis EN, Carpenter JF (2015) Concomitant Raman spectroscopy and dynamic light scattering for characterization of therapeutic proteins at high concentrations. Anal Biochem 472:7–20
Schägger H, Von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Gasteiger E, Hoogland C, Gattiker A, et al (2005) Protein identification and analysis tools on the ExPASy server. Springer
Hawe A, Sutter M, Jiskoot W (2008) Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res 25:1487–1499
Montgomerie S, Cruz JA, Shrivastava S, Arndt D, Berjanskii M, Wishart DS (2008) PROTEUS2: a web server for comprehensive protein structure prediction and structure-based annotation. Nucleic Acids Res 36:W202–W209. https://doi.org/10.1093/nar/gkn255
Bursell S-E, Yu N-T (1990) Fluorescence and Raman spectroscopy of the crystalline lens. Noninvasive Diagn Tech Ophthalmol 319–341
Rygula A, Majzner K, Marzec KM, Kaczor A, Pilarczyk M, Baranska M (2013) Raman spectroscopy of proteins: a review. J Raman Spectrosc 44:1061–1076
Benevides JM, Overman SA, Thomas GJ Jr (2003) Raman spectroscopy of proteins. Curr Protoc Protein Sci 33:17–18
Wen Z (2007) Raman spectroscopy of protein pharmaceuticals. J Pharm Sci 96:2861–2878
Lubbe L, Cozier GE, Oosthuizen D, Acharya KR, Sturrock ED (2020) ACE2 and ACE: structure-based insights into mechanism, regulation and receptor recognition by SARS-CoV. Clin Sci Lond Engl 1979 134:2851–2871. https://doi.org/10.1042/CS20200899
Zhang H, Baker A (2017) Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care Lond Engl 21:305. https://doi.org/10.1186/s13054-017-1882-z
Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW (2010) Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59:529–538. https://doi.org/10.2337/db09-1218
Tikellis C, Thomas MC (2012) Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012:256294. https://doi.org/10.1155/2012/256294
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui C-C, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436:112–116. https://doi.org/10.1038/nature03712
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586–590. https://doi.org/10.1007/s00134-020-05985-9
Tada T, Fan C, Chen JS, Kaur R, Stapleford KA, Gristick H, Dcosta BM, Wilen CB, Nimigean CM, Landau NR (2020) An ACE2 microbody containing a single immunoglobulin fc domain is a potent inhibitor of SARS-CoV-2. Cell Rep 33:108528. https://doi.org/10.1016/j.celrep.2020.108528
D’Onofrio N, Scisciola L, Sardu C, Trotta MC, De Feo M, Maiello C, Mascolo P, De Micco F, Turriziani F, Municinò E, Monetti P, Lombardi A, Napolitano MG, Marino FZ, Ronchi A, Grimaldi V, Hermenean A, Rizzo MR, Barbieri M, Franco R, Pietro Campobasso C, Napoli C, Municinò M, Paolisso G, Balestrieri ML, Marfella R (2021) Glycated ACE2 receptor in diabetes: open door for SARS-COV-2 entry in cardiomyocyte. Cardiovasc Diabetol 20:99. https://doi.org/10.1186/s12933-021-01286-7
Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279:17996–18007. https://doi.org/10.1074/jbc.M311191200
Chaudhuri K, Chatterjee SN (2009) Cholera toxin (CT): structure. Cholera toxins. Springer, Berlin, pp 105–123
Acknowledgements
The financial support of the National Institute of Genetic Engineering and Biotechnology (NIGEB) is greatly appreciated. Also, the authors appreciate and acknowledge the financial support of the Shiraz University Research Council, and Iran National Science Foundation (INSF).
Funding
This work was also partially supported by INSF (grant number 99032483).
Author information
Authors and Affiliations
Contributions
MG: Software, Formal analysis, Investigation, Data Curation, Writing-Original Draft. MBS: Writing and Editing, Software, Investigation, Data Curation. SHK: Software, Investigation, Data Curation. AN: Methodology, Investigation Validation, Data Curation, Resources. AAM-Movahedi: Writing-Review and Editing, Visualization, Supervision, Funding acquisition. RY: Conceptualization, Methodology, Validation, Resources, Writing—Review and Editing, Visualization, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghahramani, M., Shahsavani, M.B., Khaleghinejad, S.H. et al. Efficient Expression in the Prokaryotic Host System, Purification and Structural Analyses of the Recombinant Human ACE2 Catalytic Subunit as a Hybrid Protein with the B Subunit of Cholera Toxin (CTB-ACE2). Protein J 43, 24–38 (2024). https://doi.org/10.1007/s10930-023-10164-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-023-10164-y