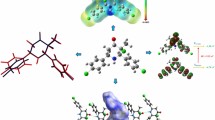
Abstract
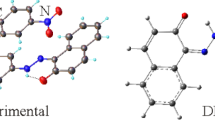
The title compound is synthesized, and characterized by spectroscopic and XRD methods. The compound crystallizes in the orthorhombic crystal system with the space group P212121. The structure exhibits C–H⋯N intermolecular interaction and π⋯π interactions. Hirshfeld surface analysis was performed to determine the individual contributions of intermolecular contacts to the crystal packing. The structural and electronic properties of the molecule were investigated by density functional theory method with B3LYP hybrid functional. Intramolecular interactions involved in the crystal structure was analyzed through topological atom-in-molecules analysis and noncovalent interactions method. Molecular electrostatic potential surface shows the chemical reactive regions around the nitrogen and hydrogen atoms.
Graphical Abstract
The article presents the characterization of synthesized compound by single crystal X-ray diffraction method. Atom-in-molecules analysis and noncovalent interactions Intramolecular interactions involved in the crystal structure were analyzed by DFT method.











Similar content being viewed by others
References
Al-Marhabi RA, Abbas HS, Ammar YA (2015) Molecules 20:19805–19822. https://doi.org/10.3390/molecules201119655
Ahmed EA, Mohamed MFA, Omran A, Salah H (2020). Synth Commun. https://doi.org/10.1080/00397911.2020.1787448
Ismail MMF, Amin KM, Noaman E, Soliman DH, Ammar YA (2010) Eur J Med Chem 45:2733–2738
Yoo HW, Lee YS, Suh ME, Kim DJ, Park SW (1998) Arch Pharm 10:331–333
Pereira JA, Pessoa AM, Cordeiro MNDS, Fernandes R, Prudencio C, Noronha JP, Vieira M (2015) Eur J Med Chem 97:664–672
El Newahie A, Nissan Y, Ismail N, El Ella AD, Khojah S, Abouzid K (2019) Molecules 24(6):1175. https://doi.org/10.3390/molecules24061175
Alswah M, Ghiaty A, El-Morsy A (2013) K El-Gamal 587054:1–7. https://doi.org/10.1155/2013/587054
Saadaoui I, Krichen F, Salah BB, Mansour R, Miled N, Bougatef A, Kossentini M (2018). J Mol Str. https://doi.org/10.1016/j.molstruc.2018.12.008
El-Zahabi SAH (2017) Arch. Pharm. Chem. Life Sci. 350:e1700028. https://doi.org/10.1002/ardp.201700028
Fabian L, Porro MT, Gómez N, Salvatori M, Turk G, Estrin D, Moglioni A (2020) Eur J Med Chem 188(111987):0223–5234. https://doi.org/10.1016/j.ejmech.2019.111987
Elzupir AO, Ali MKM, Hussein RAH, Ibrahem MA, Al-Muhanna MK, Ibnaouf KH (2018). J Mol Str. https://doi.org/10.1016/j.molstruc.2018.10.035
Montana M, Montero V, Khoumeri O, Vanelle P (2020) Molecules 25:2784. https://doi.org/10.3390/molecules25122784
Oyallon B, Botineau BM, Logé C, Robert T, Bach S, Ibrahim S, Raoul W, Croix C, Berthelot P, Guillon J, Pinaud N, Gouilleux F, Massuard MV, Sabourin CD (2021) Molecules 26:867. https://doi.org/10.3390/molecules26040867
Karzazi Y, Belghiti ME, El-Hajjaji F, Hammouti B (2016) J Mater Environ Sci 7(10):3916–3929
Kumar A, Kumar S, Saxena A, De A, Mozumdar S (2008) Catal Commun 9:778–784
Raja B, Balachandran V, Revathi B, Anitha K (2018). Mater Res Innov. https://doi.org/10.1080/14328917.2018.1477544
Dahbi S, Methnani E, Bisseret P (2010) Tetrahedron Lett 51:5516–5520
Asif M (2016) Euro Rev Chem Res 8:31–51
Arthur F, Michael L, Graham S (2012) J Org Chem 45:1909–1919
Richard JK (2003) Chem Commun 18:2286–2287
Lainne SE, Suling WJ, Reynolds RC (2002) J Med Chem 45(25):5604–5606
Kiran KR, Swaroop TR, Santhosh C, Rangappa KS, Sadashiva MP (2021) ChemistrySelect 6:1–5. https://doi.org/10.1002/slct.202102071
Bruker (2012) SAINT PLUS. Bruker AXS Inc., Madison, Wisconsin
Sheldrick GM (2008) Acta Cryst Sec A 64:112–122
Spek AL (1990) Acta Cryst Sec A 46:34
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe PE, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J App Cryst 41:466–470
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor RJ (1987) Chem Soc Perkin Trans 2(12):S1–S19
Yang XJ, Drepper F, Wu B, Sun WH, Haehnel W, Janiak C (2005) Dalton Trans 2:256–267. https://doi.org/10.1039/B414999H
Spackman MA, Jayatilaka D (2009) Cryst Eng Commun 11:19–32
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17.5
Spackman MA, McKinnon JJ (2002) Cryst Eng Comm 4(66):378–392. https://doi.org/10.1039/B203191B
Mackenzie CF, Spackman PR, Jayatilaka D, Spackman MA (2017) IUCrJ 4:575–587
Turner MJ, Thomas SP, Shi MW, Jayatilaka D, Spackman MA (2015) Chem Commun 51:3735–3738
Gaussian 09, Revision A.02, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian, Inc., Wallingford CT. https://gaussian.com
Tirado RJ, Jorgensen WL (2008) J Chem Theory Comput 4(2):297–306. https://doi.org/10.1021/ct700248k
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Tetrahedron 58(22):4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Pearson RG (2005) J Chem Sci 117(5):369–377
Kumar PSV, Raghavendra V, Subramanian V (2016) J Chem Sci 128(10):1527–1536
Lohith TN, Hema MK, Karthik CS, Sandeep S, Mallesha L, Alsaiari NS, Sridhar MA, Katubi KM, Abualnaja KM, Lokanath NK, Mallu P (2022) J Mol Stru 26:133378. https://doi.org/10.1016/j.molstruc.2022.133378
Jayappa M, Akhileshwari P, Sridhar MA, Nagarajappa L, Nagaraju S, Raghavendra S, Jayappa M (2021) Eur J Chem 12(1):69–76. https://doi.org/10.5155/eurjchem.12.1.69-76.2067
Eimre K, Urgel JI, Hayashi H, Giovannantonio MD, Ruffieux P, Sato S, Otomo S, Chan YS, Aratani N, Passerone D, Groning O, Yamada H, Fasel R, Pignedoi CA (2022) Nat Commun 13:511. https://doi.org/10.1038/s41467-022-27961-1
Laplaza R, Peccati FA, Boto R, Quan C, Carbone A, Piquemal J, Maday Y, Garcia JC (2020) J WIREs Comput Mol Sci 1497:1–18. https://doi.org/10.1002/wcms.1497
Lu T, Chen F (2012) J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten KJ (1996) J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Acknowledgements
Akhileshwari P. thanks to DST-KSTePS, Government of Karnataka, Bengaluru. Thanks to SAIF, IIT Madras, Chennai.
Author information
Authors and Affiliations
Contributions
All authors have contributed in preparation of the manuscript. PA: Conceptualization and Interpretation of the data, visualization, methodology, and writing-original draft of the manuscript. KRK: Synthesis and spectroscopic characterization. MAS: Investigation, supervision, and approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhileshwari, P., Kiran, K.R., Sridhar, M.A. et al. Crystal Structure Characterization, Interaction Energy Analysis and DFT Studies of 3-(4-Chlorophenyl)-N-phenylquinoxalin-2-amine. J Chem Crystallogr 53, 185–196 (2023). https://doi.org/10.1007/s10870-022-00959-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00959-9