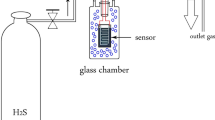
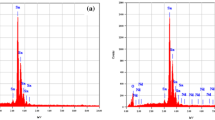
Abstract
The influence of Ag doping on the SnO2 sensor operating at room temperature is reported in this work. The SnO2 and Ag-doped SnO2 films are deposited by the nebulizer spray pyrolysis method with different Ag (1, 2, 3, and 4 wt%) concentrations. The characterization of doped thin films was done using multiple advanced techniques to understand their crystallization, morphology, optical and electrical properties to find the optimized Ag concentration on the SnO2 surface. It was found that the doping of different concentrations into the SnO2 lattice increases the preferential orientation of the peak along (211) plane up to 3wt% Ag and starts decreasing at 4 wt%. The estimated crystallite size of the 3wt% Ag-doped SnO2 thin films shows larger value of 98 nm with improved thickness and decreased strain. The morphologies of all the prepared thin films exhibits small leafy flakes structure with change in the particle size and improved agglomeration with increased Ag concentration. The optical studies showed better transparency in the visible and near-IR regions with decreased transmittance and increase in bandgap with increasing Ag concentration in SnO2. The PL results suggest that the 3wt% Ag-doped SnO2 has high-intensity emission peaks over the visible regions indicating the presence of more oxygen vacancies which acts as recombination centers to trap large amount of target gas. Finally, the gas sensing properties of the samples at different concentration of ammonia gas (i.e., 50, 100, 150, 200, and 250 ppm) are studied, which revealed that the 3wt% Ag-doped SnO2 showed better sensitivity/response of 120% with maximum response and recovery times of 32 and 17 s at 250 ppm of ammonia gas. Thus, the undoped SnO2 has been optimized with different doping amounts of Ag ions to obtain the best ammonia sensor.









Similar content being viewed by others
References
Beniwal A, Srivastava V (2019) Sol-gel assisted nano-structured SnO2 sensor for low concentration ammonia detection at room temperature. Mater Res Express 6(4):046421. https://doi.org/10.1088/2053-1591/aafdd8
Beniwal A (2019) Electrospun SnO2/PPy nanocomposite for ultra-low ammonia concentration detection at room temperature. Sens Actuators B Chem 296:126660. https://doi.org/10.1016/j.snb.2019.126660
Wen Z, Tian-mo L (2010) Gas-sensing properties of SnO2–TiO2 -based sensor for volatile organic compound gas and its sensing mechanism. Phys B Phys Condens Matter 405:1345–1348. https://doi.org/10.1016/j.physb.2009.11.086
Mitra P, Mondal S (2008) Hydrogen and LPG sensing properties of SnO2 films obtained by direct oxidation of SILAR deposited SnS, Bull Polish Acad Sci Tech Sci, 295–300
Diwan MH, Kadem WM, Khodair ZT (2019) Effect of modified SnO2 film surface on the ammonia gas sensitivity at room temperature. AIP Conf Proc. https://doi.org/10.1063/1.5123080
Jiang MH, Lu P, Lei YM, Chai YQ, Yuan R, Zhuo Y (2018) Self-accelerated electrochemiluminescence emitters of Ag@SnO2 nanoflowers for sensitive detection of cardiac troponin T. Electrochim Acta 271:464–471. https://doi.org/10.1016/j.electacta.2018.03.177
Beniwal A, Srivastava V (2019) Sunny, Sol–gel assisted nano-structured SnO2 sensor for low concentration ammonia detection at room temperature. Mater Res Express 6:046421. https://doi.org/10.1088/2053-1591/aafdd8
Yu H, Li J, Tian Y, Li Z (2018) Environmentally friendly recycling of SnO2/Sn3O4 from tin anode slime for application in formaldehyde sensing material by Ag/Ag2O modification. J Alloys Compd 765:624–634. https://doi.org/10.1016/j.jallcom.2018.06.258
Zhang L, He J, Jiao W (2019) Synthesis and gas sensing performance of NiO decorated SnO2 vertical-standing nanotubes composite thin films. Sens Actuators B Chem 281:326–334. https://doi.org/10.1016/j.snb.2018.10.121
Poloju M, Nagabandi Jayababu MV, Reddy R (2018) Improved gas sensing performance of Al doped ZnO/CuO nanocomposite based ammonia gas sensor. Mater Sci Eng B Solid-State Mater Adv Technol 227:61–67. https://doi.org/10.1016/j.mseb.2017.10.012
Khuspe GD, Navale ST, Chougule MA, Patil VB (2013) Ammonia gas sensing properties of CSA doped PANi-SnO2 nanohybrid thin films. Synth Met 185–186:1–8. https://doi.org/10.1016/j.synthmet.2013.09.032
Jazi FS, Parvin N, Tahriri M, Alizadeh M, Abedini S, Alizadeh M (2014) The relationship between the synthesis and morphology of SnO2-Ag2O nanocomposite. Synth React Inorg Met Nano-Metal Chem 44:759–764. https://doi.org/10.1080/15533174.2013.783862
Van Toan N, Hung CM, Van Duy N, Hoa ND, Le DTT, Van Hieu N (2017) Bilayer SnO2–WO3 nanofilms for enhanced NH3 gas sensing performance. Mater Sci Eng B 224:163–170. https://doi.org/10.1016/j.mseb.2017.08.004
Bhardwaj A, Kumar A, Sim U, Im HN, Song SJ (2020) Synergistic enhancement in the sensing performance of a mixed-potential NH3 sensor using SnO2@CuFe2O4 sensing electrode. Sens ActuatorsB Chem. https://doi.org/10.1016/j.snb.2020.127748
Maheswari S, Karunakaran M, Kasirajan K, Bruno Chandrasekar L, Boomi P (2020) Yttrium - substituted SnO2 thin films and its gas sensing activity against NH3 gas: characterization and sensitivity evaluation. Sens Actuators A Phys 315:112303. https://doi.org/10.1016/j.sna.2020.112303
Liu CH, Zhang L, He YJ (1997) Properties and mechanism study of Ag doped SnO2 thin films as H2S sensors. Thin Solid Films 304:13–15. https://doi.org/10.1016/S0040-6090(97)00107-7
Khamfoo K, Inyawilert K, Wisitsoraat A, Tuantranont A, Phanichphant S, Liewhiran C (2020) Formaldehyde sensor based on FSP-made AgOx-doped SnO2 nanoparticulate sensing films. Sens Actuators B Chem. https://doi.org/10.1016/j.snb.2020.127705
Kandasamy M, Seetharaman A, Sivasubramanian D, Nithya A, Jothivenkatachalam K, Maheswari N, Gopalan M, Dillibabu S, Eftekhari A (2018) Ni-doped SnO2 nanoparticles for sensing and photocatalysis. ACS Appl Nano Mater 1:5823–5836. https://doi.org/10.1021/acsanm.8b01473
Hassun HK, Hussein BH, Salman EMT, Shaban AH (2020) Photoelectric properties of SnO2: Ag/P–Si heterojunction photodetector. Energy Rep 6:46–54. https://doi.org/10.1016/j.egyr.2019.10.017
Debataraja A, Widia D, Yuliarto B (2017) Investigation of nanostructured SnO2 synthesized with polyol technique for CO gas sensor applications. Procedia Eng 170:60–64. https://doi.org/10.1016/j.proeng.2017.03.011
Abdelghani R, Shokry Hassan H, Morsi I, Kashyout AB (2019) Nano-architecture of highly sensitive SnO2–based gas sensors for acetone and ammonia using molecular imprinting technique. Sens Actuators B Chem 297:126668. https://doi.org/10.1016/j.snb.2019.126668
Yang L, Yin C, Zhang Z, Zhou J, Xu H (2017) The investigation of hydrogen gas sensing properties of SAW gas sensor based on palladium surface modified SnO2 thin film. Mater Sci Semicond Process 60:16–28. https://doi.org/10.1016/j.mssp.2016.11.042
Kamble DL, Harale NS, Patil VL, Patil PS, Kadam LD (2017) Characterization and NO2 gas sensing properties of spray pyrolyzed SnO2 thin films. J Anal Appl Pyrolysis 127:38–46. https://doi.org/10.1016/j.jaap.2017.09.004
Kang J-G, Park J-S, Lee H-J (2017) Pt-doped SnO2 thin film based micro gas sensors with high selectivity to toluene and HCHO. Sens Actuators B Chem 248:1011–1016. https://doi.org/10.1016/j.snb.2017.03.010
Zhang S, Zhang B, Sun G, Li Y, Zhang B, Wang Y, Cao J, Zhang Z (2019) One-step synthesis of Ag/SnO2/rGO nanocomposites and their trimethylamine sensing properties. Mater Res Bull 114:61–67. https://doi.org/10.1016/j.materresbull.2019.02.019
Su PG, Yang LY (2016) NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Sens Actuators B Chem 223:202–208. https://doi.org/10.1016/j.snb.2015.09.091
Mitra P, Mondal S (2008) Hydrogen and LPG sensing properties of SnO2 films obtained by direct oxidation of SILAR deposited SnS. Bull Polish Acad Sci Tech Sci 56:295–300
Tyagi P, Sharma A, Tomar M, Gupta V (2016) Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens Actuators B Chem 224:282–289. https://doi.org/10.1016/j.snb.2015.10.050
Kolhe PS, Koinkar PM, Maiti N, Sonawane KM (2017) Synthesis of Ag doped SnO2 thin films for the evaluation of H2S gas sensing properties. Phys B Condens Matter 524:90–96. https://doi.org/10.1016/j.physb.2017.07.056
Zhang J, Zhang B, Yao S, Li H, Chen C, Bala H, Zhang Z (2021) Improved triethylamine sensing properties of fish-scale-like porous SnO2 nanosheets by decorating with Ag nanoparticles. J Mater. https://doi.org/10.1016/j.jmat.2021.06.005
Das HT, Vinoth S, Thirumoorthi M, Alshahrani T, Hegazy HH, Somaily HH, Shkir M, S. AIFaify, (2021) Tuning the optical, electrical, and optoelectronic properties of CuO thin films fabricated by facile SILAR dip-coating technique for photosensing applications. J Inorg Organomet Polym Mater 31:2606–2614. https://doi.org/10.1007/s10904-021-01928-z
Thai NX, Van Duy N, Van Toan N, Hung CM, Van Hieu N, Hoa ND (2020) Effective monitoring and classification of hydrogen and ammonia gases with a bilayer Pt/SnO2 thin film sensor. Int J Hydrogen Energy 45:2418–2428. https://doi.org/10.1016/j.ijhydene.2019.11.072
Yakout SM (2019) Inclusion of cobalt reinforced Ag doped SnO2 properties: electrical, dielectric constant, magnetic and photocatalytic insights. J Mater Sci Mater Electron 30:17053–17065. https://doi.org/10.1007/S10854-019-02052-Y/FIGURES/16
Parthibavarman M, Sathishkumar S, Jayashree M, BoopathiRaja R (2019) Microwave assisted synthesis of pure and Ag doped SnO2 quantum dots as novel platform for high photocatalytic activity performance. J Clust Sci 30:351–363. https://doi.org/10.1007/S10876-018-01493-5
Xu X, Chen Y, Zhang G, Ma S, Lu Y, Bian H, Chen Q (2017) Highly sensitive VOCs-acetone sensor based on Ag-decorated SnO2 hollow nanofibers. J Alloys Compd 703:572–579. https://doi.org/10.1016/j.jallcom.2017.01.348
Singh S, Sattigeri RM, Kumar S, Jha PK, Sharma S (2021) Superior room-temperature ammonia sensing using a hydrothermally synthesized MoS2/SnO2 composite. ACS Omega 6:11602–11613. https://doi.org/10.1021/acsomega.1c00805
Kondawar SB, More AM, Sharma HJ, Dongre SP (2017) Ag-SnO2/Polyaniline composite nanofibers for low operating temperature hydrogen gas sensor. J Mater Nanosci 4:13–18
Min BK, Choi SD (2004) SnO2 thin film gas sensor fabricated by ion beam deposition. Sens Actuators B Chem 98:239–246. https://doi.org/10.1016/j.snb.2003.10.023
Ge S, Zheng H, Sun Y, Jin Z, Shan J, Wang C, Wu H, Li M, Meng F (2016) Ag/SnO2/graphene ternary nanocomposites and their sensing properties to volatile organic compounds. J Alloys Compd 659:127–131. https://doi.org/10.1016/j.jallcom.2015.11.046
Aguilar-Leyva J, Maldonado A, de la Olvera ML (2007) Gas-sensing characteristics of undoped-SnO2 thin films and Ag/SnO2 and SnO2/Ag structures in a propane atmosphere. Mater Charact 58(8–9):740–744. https://doi.org/10.1016/j.matchar.2006.11.016
Petrov VV, Nazarova TN, Korolev AN, Kopilova NF (2008) Thin sol–gel SiO2–SnOx–AgOy films for low temperature ammonia gas sensor. Sens Actuators B Chem 133(1):291–295. https://doi.org/10.1016/j.snb.2008.02.026
Acknowledgements
The authors from KKU extend their appreciation to the Research centre for Advanced Materials Science (RCAMS) King Khalid University for funding this work under grant number KKU RCAMS/G014-21.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boomashri, M., Perumal, P., Das, H.T. et al. Effect of Ag on ammonia sensing of nanostructured SnO2 films at ambient room conditions. J Mater Sci 57, 7941–7953 (2022). https://doi.org/10.1007/s10853-022-07166-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07166-z