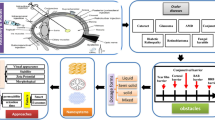
Abstract
To maintain the therapeutic drug concentration for a prolonged period of time in aqueous and vitreous humor is primary challenge for ophthalmic drug delivery. Majority of the locally administered drug into the eye is lost as to natural reflexes like blinking and lacrimation resulting in the short span of drug residence. Consequently, less than 5% of the applied drug penetrate through the cornea and reaches the intraocular tissues. The major targets for optimal ophthalmic drug delivery are increasing drug residence time in cul-de-sac of the eye, prolonging intraocular exposure, modulating drug release from the delivery system, and minimizing pre-corneal drug loss. Development of in situ gel, contact lens, intraocular lens, inserts, artificial cornea, scaffold, etc., for ophthalmic drug delivery are few approaches to achieve these major targeted objectives for delivering the drug optimally. Interpenetrating polymeric network (IPN) or smart hydrogels or stimuli sensitive hydrogels are the class of polymers that can help to achieve the targets in ophthalmic drug delivery due to their versatility, biocompatibility and biodegradability. These novel ‘‘smart” materials can alter their molecular configuration and result in volume phase transition in response to environmental stimuli, such as temperature, pH, ionic strength, electric and magnetic field. Hydrogel and tissue interaction, mechanical/tensile properties, pore size and surface chemistry of IPNs can also be modulated for tuning the drug release kinetics. Stimuli sensitive IPNs has been widely exploited to prepare in situ gelling formulations for ophthalmic drug delivery. Low refractive index hydrogel biomaterials with high water content, soft tissue-like physical properties, wettability, oxygen, glucose permeability and desired biocompatibility makes IPNs versatile candidate for contact lenses and corneal implants. This review article focuses on the exploration of these smart polymeric networks/IPNs for therapeutically improved ophthalmic drug delivery that has unfastened novel arenas in ophthalmic drug delivery.
Graphical abstract



Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Li Q et al (2021) Safety assessment of polymeric micelles as an ophthalmic drug delivery system for intravitreal administration of dasatinib. Int J Pharm 596:120226
Noreen S et al (2020) Terminalia arjuna gum/alginate in situ gel system with prolonged retention time for ophthalmic drug delivery. Int J Biol Macromol 152:1056–1067
Chen M-S et al (2008) Blood-ocular barriers. Tzu Chi Med J 20(1):25–34
Ferreira JA et al (2014) Numerical simulation of aqueous humor flow: From healthy to pathologic situations. Appl Math Comput 226:777–792
Baig MS et al (2020) Development and evaluation of cationic nanostructured lipid carriers for ophthalmic drug delivery of besifloxacin. J Drug Deliv Sci Technol 55:101496
Chan KC et al (2008) GD-DTPA enhanced MRI of ocular transport in a rat model of chronic glaucoma. Exp Eye Res 87(4):334–341
Umapathy A et al (2018) Functional characterisation of glutathione export from the rat lens. Exp Eye Res 166:151–159
Cholkar K et al (2013) Eye: anatomy, physiology and barriers to drug delivery. In: Ocular transporters and receptors. Elsevier, pp 1–36
Upadhyay M et al (2020) Oxidative stress in the retina and retinal pigment epithelium (RPE): role of aging, and DJ-1. Redox Biol 37:101623
Chu Z et al (2022) Optical coherence tomography measurements of the retinal pigment epithelium to bruch membrane thickness around geographic atrophy correlate with growth. Am J Ophthalmol 236:249–260
Khodamoradi M et al (2021) An electro-conductive hybrid scaffold as an artificial Bruch’s membrane. Mater Sci Eng C Mater Biol Appl 126:112180
Lin MC, Svitova TF (2021) Effects of model tear proteins and topical ophthalmic formulations on evaporation inhibition and biophysical property of model tear lipid nanofilm in vitro. JCIS Open 4:100028
Račić A et al (2019) Development of polysaccharide-based mucoadhesive ophthalmic lubricating vehicles: the effect of different polymers on physicochemical properties and functionality. J Drug Deliv Sci Technol 49:50–57
Silvani L et al (2020) Arabinogalactan and hyaluronic acid in ophthalmic solution: experimental effect on xanthine oxidoreductase complex as key player in ocular inflammation (in vitro study). Exp Eye Res 196:108058
Wu X-G et al (2011) The biological characteristics and pharmacodynamics of a mycophenolate mofetil nanosuspension ophthalmic delivery system in rabbits. J Pharm Sci 100(4):1350–1361
Al-Ghabeish M et al (2015) Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int J Pharm 495(2):783–791
Dave V et al (2020) Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf B Biointerfaces 194:111151
Lu GJ et al (2020) Genetically encodable contrast agents for optical coherence tomography. ACS Nano 14(7):7823–7831
Moustafa MA et al (2018) Gel in core carbosomes as novel ophthalmic vehicles with enhanced corneal permeation and residence. Int J Pharm 546(1–2):166–175
Garg V et al (2022) Topical tacrolimus progylcosomes nano-vesicles as a potential therapy for experimental dry eye syndrome. J Pharm Sci 111(2):479–484
Elmotasem H, Awad GEA (2020) A stepwise optimization strategy to formulate in situ gelling formulations comprising fluconazole-hydroxypropyl-beta-cyclodextrin complex loaded niosomal vesicles and Eudragit nanoparticles for enhanced antifungal activity and prolonged ocular delivery. Asian J Pharm Sci 15(5):617–636
Hassan N et al (2021) Doe guided chitosan based nano-ophthalmic preparation against fungal keratitis. Mater Today Proc 41:19–29
Wu B et al (2022) Flurbiprofen loaded thermosensitive nanohydrogel for ophthalmic anti-inflammatory therapy. J Drug Deliv Sci Technol 70:103253
Gudnason K, Sigurdsson S, Jonsdottir FJMB (2021) Multi-region finite element modelling of drug release from hydrogel based ophthalmic lenses. Math Biosci 331:108497
Zhang J et al (2022) Antifouling and antibacterial zwitterionic hydrogels as soft contact lens against ocular bacterial infections. Eur Polym J 167:111037
Xue Y et al (2022) Extended ocular delivery of latanoprost from niosome-laden contact lenses: In vitro characterization and in vivo studies. J Drug Deliv Sci Technol 68:103044
Kim YJ, Min JJI (2021) Property modulation of the alginate-based hydrogel via semi-interpenetrating polymer network (semi-IPN) with poly (vinyl alcohol). Int J Biol Macromol 193:1068–1077
Liu L, Sheardown HJB (2005) Glucose permeable poly (dimethyl siloxane) poly (N-isopropyl acrylamide) interpenetrating networks as ophthalmic biomaterials. Biomaterials 26(3):233–244
Yang M-C, Tran-Nguyen PLJC, Biointerfaces SB (2021) Evaluation of silicone hydrogel contact lenses based on poly (dimethylsiloxane) dialkanol and hydrophilic polymers. Colloids Surf B 206:111957
Chen F et al (2020) Simultaneous interpenetrating polymer network of collagen and hyaluronic acid as an in situ-forming corneal defect filler. Chem Mater 32(12):5208–5216
Feng L et al (2021) Thermo-gelling dendronized chitosans as biomimetic scaffolds for corneal tissue engineering. ACS Appl Mater Interfaces 13(41):49369–49379
Li J, Stachowski M, Zhang Z (2015) Application of responsive polymers in implantable medical devices and biosensors. Switch Respon Surf Mater Biomed Appl 15:259–298
Shivashankar M, Mandal BK (2012) A review on interpenetrating polymer network. Int J Phram Phram Sci 4(5):1–7
Lohani A et al (2014) Interpenetrating polymer networks as innovative drug delivery systems. J Drug Deliv 2014:528
Pal K, Paulson AT, Rousseau D (2009) Biopolymers in controlled-release delivery systems. In: Modern biopolymer science. Elsevier, pp 519–557
Wu B et al (2021) Cell penetrating peptide TAT-functionalized liposomes for efficient ophthalmic delivery of flurbiprofen: penetration and its underlying mechanism, retention, anti-inflammation and biocompatibility. Int J Pharm 598:120405
Sun X et al (2022) Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater 138:193–207
Sweeney C et al (2022) Impact of mucoadhesive agent inclusion on the intraocular pressure lowering profile of Δ9-tetrahydrocannabinol-valine-hemisuccinate loaded nanoemulsions in New Zealand white rabbits. Int J Pharm 616:121564
Chetoni P et al (1998) Silicone rubber/hydrogel composite ophthalmic inserts: preparation and preliminary in vitro/in vivo evaluation. Eur J Pharm Biopharm 46(1):125–132
Yanez F et al (2008) Macromolecule release and smoothness of semi-interpenetrating PVP–pHEMA networks for comfortable soft contact lenses. Eur J Pharm Biopharm 69(3):1094–1103
Kushwaha SK, Saxena P, Rai A (2012) Stimuli sensitive hydrogels for ophthalmic drug delivery: a review. Int J Pharm Investig 2(2):54
Hasnain MS, Nayak AK (2018) Chitosan as responsive polymer for drug delivery applications. Stimuli responsive polymeric nanocarriers for drug delivery applications, vol 1. Elsevier, pp 581–605
Gupta P, Vermani K, Garg S (2002) Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today 7(10):569–579
Wu W, Wang D-S (2010) A fast pH-responsive IPN hydrogel: Synthesis and controlled drug delivery. React Funct Polym 70(9):684–691
Wang W, Wang A (2010) Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly (sodium acrylate) and polyvinylpyrrolidone. Carbohyd Polym 80(4):1028–1036
Lim LS et al (2017) Synthesis and swelling behavior of pH-sensitive semi-IPN superabsorbent hydrogels based on poly (acrylic acid) reinforced with cellulose nanocrystals. Nanomaterials 7(11):399
Mohamadnia Z et al (2007) pH-sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J Bioact Compat Polym 22(3):342–356
Kim SJ et al (2003) Electrical/pH-sensitive swelling behavior of polyelectrolyte hydrogels prepared with hyaluronic acid–poly (vinyl alcohol) interpenetrating polymer networks. React Funct Polym 55(3):291–298
Kozhunova EY, Vyshivannaya OV, Nasimova IR (2019) “Smart” IPN microgels with different network structures: self-crosslinked vs conventionally crosslinked. Polymer 176:127–134
Morimoto N, Yamamoto MJL (2021) Design of an LCST–UCST-like thermoresponsive zwitterionic copolymer. Langmuir 37(11):3261–3269
Fu X, Xing C, Sun JJB (2020) Tunable LCST/UCST-type polypeptoids and their structure-property relationship. Biomacromol 21(12):4980–4988
Wałach W et al (2021) Alternative to poly (2-isopropyl-2-oxazoline) with a reduced ability to crystallize and physiological LCST. Int J Mol Sci 22(4):2221
d’Oliveira H et al (2017) Test-area surface tension calculation of the graphene-methane interface: fluctuations and commensurability. J Chem Phys 146(21):214112
Liu R et al (2022) Preparation of LCST regulable DES-lignin-g-PNVCL thermo-responsive polymer by ARGET-ATRP. Int J Biol Macromol 194:358–365
Xie B et al (2015) An injectable thermosensitive polymeric hydrogel for sustained release of Avastin® to treat posterior segment disease. Int J Pharm 490(1–2):375–383
Cao Y et al (2007) Poly (N-isopropylacrylamide)–chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release 120(3):186–194
Jung SW et al (2018) Multivalent ion-based in situ gelling polysaccharide hydrogel as an injectable bone graft. Carbohyd Polym 180:216–225
Tsuru T, Sugimura K, Nishio YJ (2017) Superparamagnetic IPN gels of carrageenan/PHEMA excelling in shape retention. Carbohyd Polym 178:1–7
Wang W-B et al (2013) One-step in situ fabrication of a granular semi-IPN hydrogel based on chitosan and gelatin for fast and efficient adsorption of Cu2+ ion. Colloids Surf B Biointerfaces 106:51–59
Jana S et al (2015) Metal ion-induced alginate–locust bean gum IPN microspheres for sustained oral delivery of aceclofenac. Int J Biol Macromol 72:47–53
Zeng L et al (2020) Anion exchange membrane based on interpenetrating polymer network with ultrahigh ion conductivity and excellent stability for alkaline fuel cell. Research 2020:5248
Singha NR et al (2017) Synthesis of guar gum-g-(acrylic acid-co-acrylamide-co-3-acrylamido propanoic acid) IPN via in situ attachment of acrylamido propanoic acid for analyzing superadsorption mechanism of Pb (II)/Cd (II)/Cu (II)/MB/MV. Polym Chem 8(44):6750–6777
Loghin DFA et al (2017) Preparation and characterization of oxidized starch/poly (N, N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int J Biol Macromol 96:589–599
Hu X et al (2015) Mechanically tough biomacromolecular IPN hydrogel fibers by enzymatic and ionic crosslinking. Int J Biol Macromol 72:403–409
Paulsson M, Hägerström H, Edsman K (1999) Rheological studies of the gelation of deacetylated gellan gum (Gelrite®) in physiological conditions. Eur J Pharm Sci 9(1):99–105
Shelley H et al (2018) In situ gel formulation for enhanced ocular delivery of nepafenac. J Pharm Sci 107(12):3089–3097
Krtalić I et al (2018) D-optimal design in the development of rheologically improved in situ forming ophthalmic gel. J Pharm Sci 107(6):1562–1571
Fang G et al (2021) Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater Sci Eng C 127:112212
Elbahwy IA et al (2018) Mucoadhesive self-emulsifying delivery systems for ocular administration of econazole. Int J Pharm 541(1–2):72–80
Del Amo EM, Urtti A (2008) Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discov Today 13(3–4):135–143
Rafie F et al (2010) In vivo evaluation of novel nanoparticles containing dexamethasone for ocular drug delivery on rabbit eye. Curr Eye Res 35(12):1081–1089
Choi SW, Kim J (2018) Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: a review. Materials 11(7):1125
Awwad S et al (2019) In situ antibody-loaded hydrogel for intravitreal delivery. Eur J Pharm Sci 137:104993
Al-Kinani AA et al (2018) Ophthalmic gels: past, present and future. Adv Drug Deliv Rev 126:113–126
del Amo EM et al (2015) Intravitreal clearance and volume of distribution of compounds in rabbits: in silico prediction and pharmacokinetic simulations for drug development. Eur J Pharm Biopharm 95:215–226
Hou Y et al (2019) Ultra-small micelles based on polyoxyl 15 hydroxystearate for ocular delivery of myricetin: optimization, in vitro, and in vivo evaluation. Drug Deliv 26(1):158–167
Myung D et al (2009) Bioactive interpenetrating polymer network hydrogels that support corneal epithelial wound healing. J Biomed Mater Res A 90(1):70–81
Rodriguez-Tenreiro C et al (2007) Cyclodextrin/carbopol micro-scale interpenetrating networks (ms-IPNs) for drug delivery. J Control Release 123(1):56–66
Agrawal AK, Das M, Jain S (2012) In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv 9(4):383–402
Lin H-R, Sung K, Vong W-J (2004) In situ gelling of alginate/pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromol 5(6):2358–2365
Egbu R et al (2018) Antibody loaded collapsible hyaluronic acid hydrogels for intraocular delivery. Eur J Pharm Biopharm
Jiang L et al (2019) Scaffold hopping-driven optimization of 4-(quinazolin-4-yl)-3, 4-dihydroquinoxalin-2 (1 H)-ones as novel tubulin inhibitors. ACS Med Chem Lett 11(1):83–89
Bhardwaj V, Harit G, Kumar S (2012) Interpenetrating polymer network (IPN): novel approach in drug delivery. Int J Drug Dev Res 4(3):41–54
Myung D et al (2008) Development of hydrogel-based keratoprostheses: a materials perspective. Biotechnol Prog 24(3):735–741
Elkhouly H, Mamdouh W, El-Korashy DIJ (2021) Electrospun nano-fibrous bilayer scaffold prepared from polycaprolactone/gelatin and bioactive glass for bone tissue engineering. J Mater Sci Mater Med 32(9):1–15
Wendland RJ et al (2021) The effect of retinal scaffold modulus on performance during surgical handling. Exp Eye Res 207:108566
Sharma R et al (2019) Clinical-grade stem cell–derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med 11(475):eaat5580
Weiss MD et al (2006) Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry 45(5):512–519
Albon J (2003) Corneal transplantation and the artificial cornea. J Mech Med Biol 3(01):95–106
Al-Ani A et al (2021) Scaffold-free retinal pigment epithelium microtissues exhibit increased release of PEDF. Int J Mol Sci 22(21):11317
Polisetti N et al (2021) A decellularized human corneal scaffold for anterior corneal surface reconstruction. Sci Rep 11(1):1–15
Soleimannejad M et al (2018) Fibrin gel as a scaffold for photoreceptor cells differentiation from conjunctiva mesenchymal stem cells in retina tissue engineering. Artif Cells Nanomed Biotechnol 46(4):805–814
Hotaling NA et al (2016) Nanofiber scaffold-based tissue-engineered retinal pigment epithelium to treat degenerative eye diseases. J Ocul Pharmacol Ther 32(5):272–285
Brunette I et al (2017) Alternatives to eye bank native tissue for corneal stromal replacement. Prog Retin Eye Res 59:97–130
Chung C-W et al (2011) Interpenetrating polymer network (IPN) scaffolds of sodium hyaluronate and sodium alginate for chondrocyte culture. Colloids Surf B Biointerfaces 88(2):711–716
Griffith M et al. Biomimetic corneal substitutes for transplantation: from benchtop to bedside
Liu W et al (2009) Collagen–phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials 30(8):1551–1559
Hartmann L et al (2011) Toward the development of an artificial cornea: improved stability of interpenetrating polymer networks. J Biomed Mater Res B Appl Biomater 98(1):8–17
Deng C et al (2010) Collagen and glycopolymer based hydrogel for potential corneal application. Acta Biomater 6(1):187–194
Chirila TV et al (1998) Hydrophilic sponges based on 2-hydroxyethyl methacrylate. VI. Effect of phase sequence inversion on the characteristics of IPN between sponges and homogeneous gels. J Mater Sci Mater Med 40(1–2):97–104
Hicks CR et al (1996) Keratoprosthesis: preliminary results of an artificial corneal button as a full-thickness implant in the rabbit model. Aust N Z J Ophthalmol 24(3):297–303
Myung D et al (2008) Glucose-permeable interpenetrating polymer network hydrogels for corneal implant applications: a pilot study. Curr Eye Res 33(1):29–43
Zhang Q et al (2012) High refractive index inorganic–organic interpenetrating polymer network (IPN) hydrogel nanocomposite toward artificial cornea implants. ACS Macro Lett 1(7):876–881
Parke-Houben R et al (2015) Interpenetrating polymer network hydrogel scaffolds for artificial cornea periphery. J Mater Sci Mater Med 26(2):1–12
Liu J, Wang XJAP (2022) Ofloxacin-loaded niosome-laden contact lens: improved properties of biomaterial for ocular drug delivery. AAPS PharmSciTech 23(1):1–9
Ding X et al (2020) Soft contact lens with embedded microtubes for sustained and self-adaptive drug delivery for glaucoma treatment. ACS Appl Mater Interfaces 12(41):45789–45795
Wei Y et al (2020) Design of circular-ring film embedded contact lens for improved compatibility and sustained ocular drug delivery. Eur J Pharm Biopharm 157:28–37
Zhu Q et al (2018) Inner layer-embedded contact lenses for ion-triggered controlled drug delivery. Mater Sci Eng C Mater Biol Appl 93:36–48
Zhu Q et al (2018) Inner layer-embedded contact lenses for pH-triggered controlled ocular drug delivery. Eur J Pharm Biopharm 128:220–229
Pimenta AF et al (2016) Diffusion-based design of multi-layered ophthalmic lenses for controlled drug release. PLoS ONE 11(12):e0167728
Xu J et al (2014) Simultaneous interpenetrating silicone hydrogel based on radical/addition polymerization for extended release of ocular therapeutics. J Biomater Sci Polym Ed 25(2):121–135
Karlgard C et al (2003) In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int J Pharm 257(1–2):141–151
Zheng Y, Zheng SJR, Polymers F (2012) Poly (ethylene oxide)-grafted poly (N-isopropylacrylamide) networks: preparation, characterization and rapid deswelling and reswelling behavior of hydrogels. React Funct Polym 72(3):176–184
Shimizu T et al (2010) Super-hydrophilic silicone hydrogels with interpenetrating poly (2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials 31(12):3274–3280
McElroy DM et al (2014) The effect of photoinitiator concentration on the physicochemical properties of hydrogel contact lenses. Appl Mech Mater
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SR: Writing original draft, reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rathod, S. Interpenetrating polymeric network (IPNs) in ophthalmic drug delivery: Breaking the barriers. Int Ophthalmol 43, 1063–1074 (2023). https://doi.org/10.1007/s10792-022-02482-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02482-4