Abstract
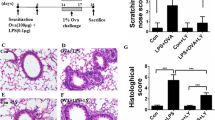
Acute lung injury (ALI) is a life-threatening disorder stemmed mainly from an uncontrolled inflammatory response. Lipopolysaccharide (LPS) is commonly used to induce ALI animal models. Toll-like receptor 4 (TLR4) is the main receptor for LPS, and myeloid differentiation factor 88 (MyD88) is a key adaptor protein molecule in the Toll-like receptor (TLR) signaling pathway. Thus, MyD88 knockdown heterozygous mice (MyD88+/−) were used to investigate the effect of incomplete knockout of the MyD88 gene on indirect LPS-induced ALI through intraperitoneal injection of LPS. The LPS-induced ALI significantly upregulated MyD88 expression, and heterozygous mice with incomplete knockout of the MyD88 gene (MyD88+/−) ameliorated LPS-induced histopathological injury and collagen fiber deposition. Heterozygous mice with incomplete knockout of the MyD88 gene (MyD88+/−) inhibited LPS-induced nuclear factor-κB (NF-κB) pathway activation, but TLR-4 expression tended to be upregulated. Incomplete knockdown of the MyD88 gene also downregulated LPS-induced expression of IL1-β, IL-6, TNF-α, TGF-β, SMAD2, and α-SMA. The transcriptome sequencing also revealed significant changes in LPS-regulated genes (such as IL-17 signaling pathway genes) after the incomplete knockdown of MyD88. In conclusion, this paper clarified that LPS activates the downstream NF-κB pathway depending on the MyD88 signaling pathway, which induces the secretion of inflammatory cytokines such as IL-1β/IL-6/TNF-α and ultimately triggers ALI. Incomplete knockdown of the MyD88 reverses LPS-induced lung fibrosis, which confirmed the vital role of MyD88 in LPS-induced ALI.






Similar content being viewed by others
Data Availability
This study did not include data deposited in external repositories. All relevant data are available in the manuscript.
References
Matuschak, G.M., and A.J. Lechner. 2010. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Missouri Medicine 107 (4): 252–258.
Mokra, D. 2020. Acute lung injury - from pathophysiology to treatment. Physiological Research 69 (Suppl 3): S353–S366.
Matthay, M.A., and G.A. Zimmerman. 2005. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. American Journal of Respiratory Cell and Molecular Biology 33 (4): 319–327.
Herold, S., N.M. Gabrielli, and I. Vadasz. 2013. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. American Journal of Physiology. Lung Cellular and Molecular Physiology 305 (10): L665-681.
Dengler, V., G.P. Downey, R.M. Tuder, H.K. Eltzschig, and E.P. Schmidt. 2013. Neutrophil intercellular communication in acute lung injury. Emerging roles of microparticles and gap junctions. American Journal of Respiratory Cell and Molecular Biology 49 (1): 1–5.
Densmore, J.C., P.R. Signorino, J. Ou, O.A. Hatoum, J.J. Rowe, Y. Shi, S. Kaul, D.W. Jones, R.E. Sabina, K.A. Pritchard Jr., et al. 2006. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 26 (5): 464–471.
Lee, W.L., and G.P. Downey. 2001. Neutrophil activation and acute lung injury. Current Opinion in Critical Care 7 (1): 1–7.
Mowery, N.T., W.T.H. Terzian, and A.C. Nelson. 2020. Acute lung injury. Current Problems in Surgery 57 (5): 100777.
Litvak, V., S.A. Ramsey, A.G. Rust, D.E. Zak, K.A. Kennedy, A.E. Lampano, M. Nykter, I. Shmulevich, and A. Aderem. 2009. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nature Immunology 10 (4): 437–443.
Medzhitov, R., and T. Horng. 2009. Transcriptional control of the inflammatory response. Nature Reviews Immunology 9 (10): 692–703.
Buchholz, B.M., T.R. Billiar, and A.J. Bauer. 2010. Dominant role of the MyD88-dependent signaling pathway in mediating early endotoxin-induced murine ileus. American Journal of Physiology. Gastrointestinal and Liver Physiology 299 (2): G531-538.
Wong, D.V., R.C. Lima-Junior, C.B. Carvalho, V.F. Borges, C.W. Wanderley, A.X. Bem, C.A. Leite, M.A. Teixeira, G.L. Batista, R.L. Silva, et al. 2015. The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS ONE 10 (10): e0139985.
Chen, L., L. Zheng, P. Chen, and G. Liang. 2020. Myeloid differentiation primary response protein 88 (MyD88): the central hub of TLR/IL-1R signaling. Journal of Medicinal Chemistry 63 (22): 13316–13329.
Bagchi, A., E.A. Herrup, H.S. Warren, J. Trigilio, H.S. Shin, C. Valentine, and J. Hellman. 2007. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. The Journal of Immunology 178 (2): 1164–1171.
Jiang, S., X. Li, N.J. Hess, Y. Guan, and R.I. Tapping. 2016. TLR10 is a negative regulator of both MyD88-dependent and -independent TLR signaling. The Journal of Immunology 196 (9): 3834–3841.
Funami, K., M. Sasai, Y. Ohba, H. Oshiumi, T. Seya, and M. Matsumoto. 2007. Spatiotemporal mobilization of Toll/IL-1 receptor domain-containing adaptor molecule-1 in response to dsRNA. The Journal of Immunology 179 (10): 6867–6872.
Gay, N.J., M.F. Symmons, M. Gangloff, and C.E. Bryant. 2014. Assembly and localization of Toll-like receptor signalling complexes. Nature Reviews Immunology 14 (8): 546–558.
Fitzgerald, K.A., E.M. Palsson-McDermott, A.G. Bowie, C.A. Jefferies, A.S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M.T. Harte, et al. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413 (6851): 78–83.
Ohnishi, H., H. Tochio, Z. Kato, T. Kimura, H. Hiroaki, N. Kondo, and M. Shirakawa. 2010. 1H, 13C, and 15N resonance assignment of the TIR domain of human MyD88. Biomolecular NMR Assignments 4 (2): 123–125.
Motshwene, P.G., M.C. Moncrieffe, J.G. Grossmann, C. Kao, M. Ayaluru, A.M. Sandercock, C.V. Robinson, E. Latz, and N.J. Gay. 2009. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. Journal of Biological Chemistry 284 (37): 25404–25411.
Wang, Q., R. Dziarski, C.J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2–>MyD88–>IRAK–>TRAF–>NIK–>IKK–>NF-kappaB signal transduction pathway that induces transcription of interleukin-8. Infection and Immunity 69 (4): 2270–2276.
Chow, J.C., D.W. Young, D.T. Golenbock, W.J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. Journal of Biological Chemistry 274 (16): 10689–10692.
Nativel, B., D. Couret, P. Giraud, O. Meilhac, C.L. d’Hellencourt, W. Viranaicken, and C.R. Da Silva. 2017. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Science and Reports 7 (1): 15789.
Yang, H., D.W. Young, F. Gusovsky, and J.C. Chow. 2000. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4: MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. Journal of Biological Chemistry 275 (27): 20861–20866.
Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. The Journal of Immunology 162 (7): 3749–3752.
Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11 (4): 443–451.
Wang, P., X. Han, B. Mo, G. Huang, and C. Wang. 2017. LPS enhances TLR4 expression and IFNgamma production via the TLR4/IRAK/NFkappaB signaling pathway in rat pulmonary arterial smooth muscle cells. Molecular Medicine Reports 16 (3): 3111–3116.
Hagar, J.A., D.A. Powell, Y. Aachoui, R.K. Ernst, and E.A. Miao. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341 (6151): 1250–1253.
Johnson, E.R., and M.A. Matthay. 2010. Acute lung injury: epidemiology, pathogenesis, and treatment. Journal of Aerosol Medicine and Pulmonary Drug Delivery 23 (4): 243–252.
Andonegui, G., S.M. Goyert, and P. Kubes. 2002. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels. The Journal of Immunology 169 (4): 2111–2119.
He, Z., Y. Zhu, and H. Jiang. 2009. Toll-like receptor 4 mediates lipopolysaccharide-induced collagen secretion by phosphoinositide3-kinase-Akt pathway in fibroblasts during acute lung injury. Journal of Receptor and Signal Transduction Research 29 (2): 119–125.
Janga, H., L. Cassidy, F. Wang, D. Spengler, S. Oestern-Fitschen, M.F. Krause, A. Seekamp, A. Tholey, and S. Fuchs. 2018. Site-specific and endothelial-mediated dysfunction of the alveolar-capillary barrier in response to lipopolysaccharides. Journal of Cellular and Molecular Medicine 22 (2): 982–998.
Imai, Y., K. Kuba, G.G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo, M. Ermolaeva, R. Veldhuizen, Y.H. Leung, H. Wang, et al. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133 (2): 235–249.
Chao, H.H., P.Y. Chen, W.R. Hao, W.P. Chiang, T.H. Cheng, S.H. Loh, Y.M. Leung, J.C. Liu, J.J. Chen, and L.C. Sung. 2017. Lipopolysaccharide pretreatment increases protease-activated receptor-2 expression and monocyte chemoattractant protein-1 secretion in vascular endothelial cells. Journal of Biomedical Science 24 (1): 85.
He, Z., Y. Gao, Y. Deng, W. Li, Y. Chen, S. Xing, X. Zhao, J. Ding, and X. Wang. 2012. Lipopolysaccharide induces lung fibroblast proliferation through Toll-like receptor 4 signaling and the phosphoinositide3-kinase-Akt pathway. PLoS ONE 7 (4): e35926.
Alam, S., S. Javor, M. Degardin, D. Ajami, M. Rebek, T.L. Kissner, D.M. Waag, J. Rebek Jr., and K.U. Saikh. 2015. Structure-based design and synthesis of a small molecule that exhibits anti-inflammatory activity by inhibition of MyD88-mediated signaling to bacterial toxin exposure. Chemical Biology & Drug Design 86 (2): 200–209.
Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9 (1): 143–150.
Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11 (1): 115–122.
Lord, K.A., B. Hoffman-Liebermann, and D.A. Liebermann. 1990. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene 5 (7): 1095–1097.
Wesche, H., W.J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7 (6): 837–847.
Cao, F., C. Wang, D. Long, Y. Deng, K. Mao, and H. Zhong. 2021. Network-based integrated analysis of transcriptomic studies in dissecting gene signatures for LPS-induced acute lung injury. Inflammation 44 (6): 2486–2498.
Author information
Authors and Affiliations
Contributions
Hui Fan and Yanni Wang designed the experiments and completed the manuscript. Kaochang Zhao, Li Su, and Chong Deng carried out the experiments. Guozhong Chen analyzed the data, and Jie Huang contributed to the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Laboratory Animal Management Committee of Chongqing Liangjiang Chuangxiang Medical Inspection and Certification Technology Co., Ltd. (No. 2022001). All experiments were performed in accordance with the National Institutes of Health guidelines.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, H., Wang, Y., Zhao, K. et al. Incomplete Knockdown of MyD88 Inhibits LPS-Induced Lung Injury and Lung Fibrosis in a Mouse Model. Inflammation 46, 2276–2288 (2023). https://doi.org/10.1007/s10753-023-01877-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01877-4