Abstract—
Acute kidney injury (AKI) is one of the most common diseases in patients treated in intensive care units. This study was intended to explore the underlying mechanism by which ulinastatin (UTI) influenced the inflammation and apoptosis of renal tubular epithelial cells, HK-2.
The effects of UTI on the cell viability of HK-2 cells were first measured by MTT and lactate dehydrogenase (LDH) detection kit. The apoptosis and inflammation of HK-2 cells were then determined by TUNEL, western blot, ELISA, and RT-qPCR. Then, the proteins in the Toll-like receptor 4 (TLR4)/nuclear factor κB (NF-κB) and nuclear factor erythroid 2–related factor 2 (Nrf2)/Heme oxygenase 1 (HO-1) signaling pathways were measured by western blot for confirming the relationship between UTI and these pathways. Finally, Nrf-2 inhibitor ML385 and TLR4 activator CCL-34 were respectively used on LPS-induced HK-2 cells exposed to UTI for the conduction of gain-of-function and loss-of-function assays.
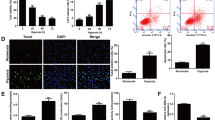
UTI treatment boosted the cell viability of HK-2 cells damaged by LPS. Furthermore, UTI exposure cut down the apoptosis rate and inhibited the expression inflammatory factors of HK-2 cells induced by LPS. UTI treatment decreased the expression of proteins in the TLR4/NF-κB pathway, increased the HO-1 expression, and prompted the translocation of Nrf2 from the cytoplasm to the nucleus. The alleviated effects of UTI on inflammation and apoptosis LPS-induced HK-2 cells were abolished by ML385 and TLR4, respectively.
UTI attenuates LPS-induced inflammation and inhibits endoplasmic reticulum stress–induced apoptosis in renal tubular epithelial cells by regulating TLR4/NF-κB and Nrf2/HO-1 pathways.





Similar content being viewed by others
Data Availability
The experimental data will be available on request.
References
Goyal A., Daneshpajouhnejad P., Hashmi MF., Bashir K., John BK. Acute kidney injury (Nursing). StatPearls. Treasure Island (FL), 2021.
Brandenburger, T., A. Salgado Somoza, Y. Devaux, and J.M. Lorenzen. 2018. Noncoding RNAs in acute kidney injury. Kidney International 94: 870–881.
Fan, P.C., C.C. Chen, Y.C. Chen, Y.S. Chang, and P.H. Chu. 2016. MicroRNAs in acute kidney injury. Human Genomics 10: 29.
Zhou, P., Z. Chen, Y. Zou, and X. Wan. 2016. Roles of non-coding RNAs in acute kidney injury. Kidney & Blood Pressure Research 41: 757–769.
Chen, C.C., Z.M. Liu, H.H. Wang, W. He, Y. Wang, and W.D. Wu. 2004. Effects of ulinastatin on renal ischemia-reperfusion injury in rats. Acta Pharmacologica Sinica 25: 1334–1340.
Feng, M., Y. Shu, Y. Yang, X. Zheng, R. Li, Y. Wang, et al. 2014. Ulinastatin attenuates experimental autoimmune encephalomyelitis by enhancing anti-inflammatory responses. Neurochemistry International 64: 64–72.
Lv, H., X. Wei, X. Yi, J. Liu, P. Lu, M. Zhou, et al. 2020. High-dose ulinastatin to prevent late-onset acute renal failure after orthotopic liver transplantation. Renal Failure 42: 137–145.
Wang X, Xue Q, Yan F, Liu J, Li S, Hu S. Ulinastatin protects against acute kidney injury in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. PLoS One 2015; 10:e0144516.
Zhao, P., L. Zhang, L. Gao, Q. Ding, Q. Yang, and J. Kuai. 2020. Ulinastatin attenuates lipopolysaccharide-induced cardiac dysfunction by inhibiting inflammation and regulating autophagy. Experimental and Therapeutic Medicine 20: 1064–1072.
Qi AL, Wu Y, Dong N, Chai YF, Zhu XM, Yao YM. Recombinant human ulinastatin improves immune dysfunction of dendritic cells in septic mice by inhibiting endoplasmic reticulum stress-related apoptosis. Int Immunopharmacol 2020; 85:106643.
Liang, S., P. Lai, X. Li, J. Xu, Y. Bao, Y. Fang, et al. 2019. Ulinastatin reduces the severity of intestinal damage in the neonatal rat model of necrotizing enterocolitis. Medical Science Monitor 25: 9123–9130.
Yu Z, Wang J, Zhang P, Ding W. Ulinastatin attenuates vascular endothelial cell damage in pregnant women with severe pre-eclampsia. An Acad Bras Cienc 2019; 91:e20180746.
Lepedda, A.J., P. De Muro, G. Capobianco, and M. Formato. 2020. Role of the small proteoglycan bikunin in human reproduction. Hormones (Athens, Greece) 19: 123–133.
Hung, L.C., C.C. Lin, S.K. Hung, B.C. Wu, M.D. Jan, S.H. Liou, et al. 2007. A synthetic analog of alpha-galactosylceramide induces macrophage activation via the TLR4-signaling pathways. Biochemical pharmacology 73: 1957–1970.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408.
Lun, M.H., X.Y. Jin, M.Y. Wang, Z. Cai, W. Du, and Z.Q. Huang. 2020. Ulinastatin improves myocardial ischemia-reperfusion injury in rats through endoplasmic reticulum stress-induced apoptosis pathway. European Review for Medical and Pharmacological Sciences 24: 5742–5749.
Lv, B., X.M. Jiang, D.W. Wang, J. Chen, D.F. Han, and X.L. Liu. 2020. Protective effects and mechanisms of action of ulinastatin against cerebral ischemia-reperfusion injury. Current Pharmaceutical Design 26: 3332–3340.
Chen Y, Xu Z, Song Q, Wang Z, Ji Z, Qiu Z, et al. [Mechanism of ulinastatin in reducing lung inflammatory injury in rats with hemorrhagic shock]. Nan Fang Yi Ke Da Xue Xue Bao 2019; 39:1232–1238.
An, H., C. Qian, and X. Cao. 2010. Regulation of Toll-like receptor signaling in the innate immunity. Sci China Life Sci 53: 34–43.
Hayden, M.S., and S. Ghosh. 2008. Shared principles in NF-kappaB signaling. Cell 132: 344–362.
Guijarro-Munoz, I., M. Compte, A. Alvarez-Cienfuegos, L. Alvarez-Vallina, and L. Sanz. 2014. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. Journal of Biological Chemistry 289: 2457–2468.
Cao, C., C. Yin, Y. Chai, H. Jin, L. Wang, and S. Shou. 2018. Ulinastatin mediates suppression of regulatory T cells through TLR4/NF-kappaB signaling pathway in murine sepsis. International Immunopharmacology 64: 411–423.
Laveti, D., M. Kumar, R. Hemalatha, R. Sistla, V.G. Naidu, V. Talla, et al. 2013. Anti-inflammatory treatments for chronic diseases: A review. Inflammation & Allergy: Drug Targets 12: 349–361.
Lim, S.M., J.J. Jeong, G.D. Kang, K.A. Kim, H.S. Choi, and D.H. Kim. 2015. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-kappaB and MAPK activation and restoring Th17/Treg cell balance. International Immunopharmacology 25: 493–503.
Lin, Y.S., L.D. Huang, C.H. Lin, P.H. Huang, Y.J. Chen, F.H. Wong, et al. 2011. In vitro and in vivo anticancer activity of a synthetic glycolipid as Toll-like receptor 4 (TLR4) activator. Journal of Biological Chemistry 286: 43782–43792.
Janssen-Heininger, Y.M., M.E. Poynter, and P.A. Baeuerle. 2000. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radical Biology & Medicine 28: 1317–1327.
Liu, X., Q. Zhu, M. Zhang, T. Yin, R. Xu, W. Xiao, et al. 2018. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxidative Medicine and Cellular Longevity 2018: 7161592.
Li, Z., Q.Q. Ma, Y. Yan, F.D. Xu, X.Y. Zhang, W.Q. Zhou, et al. 2016. Edaravone attenuates hippocampal damage in an infant mouse model of pneumococcal meningitis by reducing HMGB1 and iNOS expression via the Nrf2/HO-1 pathway. Acta Pharmacologica Sinica 37: 1298–1306.
Liu, X.H., X.L. Wang, H. Xin, D. Wu, X.M. Xin, L. Miao, et al. 2015. Induction of heme oxygenase-1 by sodium 9-hydroxyltanshinone IIA sulfonate derivative contributes to inhibit LPS-mediated inflammatory response in macrophages. Cellular Physiology and Biochemistry 36: 1316–1330.
Wang, N., F. Zhang, L. Yang, J. Zou, H. Wang, K. Liu, et al. 2017. Resveratrol protects against L-arginine-induced acute necrotizing pancreatitis in mice by enhancing SIRT1-mediated deacetylation of p53 and heat shock factor 1. International Journal of Molecular Medicine 40: 427–437.
Shiraishi, F., L.M. Curtis, L. Truong, K. Poss, G.A. Visner, K. Madsen, et al. 2000. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. American Journal of Physiology. Renal Physiology 278: F726–F736.
Cai, Z.Y., Z.X. Sheng, and H. Yao. 2017. Pachymic acid ameliorates sepsis-induced acute kidney injury by suppressing inflammation and activating the Nrf2/HO-1 pathway in rats. European Review for Medical and Pharmacological Sciences 21: 1924–1931.
Landis, R.C., K.R. Quimby, and A.R. Greenidge. 2018. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Current Pharmaceutical Design 24: 2241–2249.
Tebay, L.E., H. Robertson, S.T. Durant, S.R. Vitale, T.M. Penning, A.T. Dinkova-Kostova, et al. 2015. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radical Biology & Medicine 88: 108–146.
Author information
Authors and Affiliations
Contributions
Feixiang Chen: project development, data analysis and collection, manuscript writing.
Jiadong Zhu: project development, data analysis and collection, manuscript writing.
Wei Wang: project development, manuscript editing.
Corresponding author
Ethics declarations
Consent for Publication.
All authors have read the manuscript and agreed to submit it.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, F., Zhu, J. & Wang, W. Ulinastatin Attenuates LPS-Induced Inflammation and Inhibits Endoplasmic Reticulum Stress–Induced Apoptosis in Renal Tubular Epithelial Cells via Regulation of the TLR4/NF-κB and Nrf2/HO-1 Pathways. Inflammation 44, 2323–2332 (2021). https://doi.org/10.1007/s10753-021-01505-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01505-z