Abstract
Purpose
P53 is one of the key tumor suppressors. In normal cells, p53 is maintained at low levels by the ubiquitination of the ubiquitinated ligase MDM2. In contrast, under stress conditions such as DNA damage and ischemia, the interaction between p53 and MDM2 is blocked and activated by phosphorylation and acetylation, thereby mediating the trans-activation of p53 through its target genes to regulate a variety of cellular responses. Previous studies have shown that the expression of p53 is negligible in normal myocardium, tends to increase in myocardial ischemia and is maximally induced in ischemia-reperfused myocardium, demonstrating a possible key role of p53 in the development of MIRI. In this review, we detail and summarize recent studies on the mechanism of action of p53 in MIRI and describe the therapeutic agents targeting the relevant targets to provide new strategies for the prevention and treatment of MIRI.
Methods
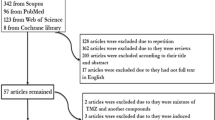
We collected 161 relevant papers mainly from Pubmed and Web of Science (search terms "p53" and "myocardial ischemia-reperfusion injury"). After that, we selected pathway studies related to p53 and classified them according to their contents. We eventually analyzed and summarized them.
Results and conclusion
In this review, we detail and summarize recent studies on the mechanism of action of p53 in MIRI and validate its status as an important intermediate affecting MIRI. On the one hand, p53 is regulated and modified by multiple factors, especially non-coding RNAs; on the other hand, p53 regulates apoptosis, programmed necrosis, autophagy, iron death and oxidative stress in MIRI through multiple pathways. More importantly, several studies have reported medications targeting p53-related therapeutic targets. These medications are expected to be effective options for the alleviation of MIRI, but further safety and clinical studies are needed to convert them into clinical applications.





Similar content being viewed by others
Data availability
Not applicable.
References
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99(4):1765–817.
Du J, Li Y, Zhao W. Autophagy and Myocardial Ischemia. Adv Exp Med Biol. 2020;1207:217–22.
Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315(6):H1553–H68.
Zhao W, Wu Y, Ye F, et al. Tetrandrine ameliorates myocardial ischemia reperfusion injury through miR-202-5p/TRPV2. Biomed Res Int. 2021;2021:8870674.
Sun MY, Li LP. MiR-140-5p targets BCL2L1 to promote cardiomyocyte apoptosis. Eur Rev Med Pharmacol Sci. 2021;25(1):1.
Ghafouri-Fard S, Shoorei H, Taheri M. Non-coding RNAs participate in the ischemia-reperfusion injury. Biomed Pharmacother. 2020;129:110419.
Hu J, Cao J, Topatana W, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol. 2021;14(1):157.
Beckerman R, Prives C. Transcriptional regulation by p53. Cold Spring Harb Perspect Biol. 2010;2(8):a000935.
Kawauchi K, Wolf SJ. Understanding p53: new insights into tumor suppression. Expert Rev Anticancer Ther. 2014;14(10):1101–3.
Maulik N, Sasaki H, Addya S, Das DK. Regulation of cardiomyocyte apoptosis by redox-sensitive transcription factors. FEBS Lett. 2000;485(1):7–12.
Yano T, Abe K, Tanno M, et al. Does p53 inhibition suppress myocardial ischemia-reperfusion injury? J Cardiovasc Pharmacol Ther. 2018;23(4):350–7.
Liu Y, Tavana O, Gu W. p53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019;11(7):564–77.
Joerger AC, Fersht AR. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu Rev Biochem. 2016;85:375–404.
Wang L, He G, Zhang P, et al. Interplay between MDM2, MDMX, Pirh2 and COP1: the negative regulators of p53. Mol Biol Rep. 2011;38(1):229–36.
Song Y, Liu Y, Pan S, et al. Role of the COP1 protein in cancer development and therapy. Semin Cancer Biol. 2020;67(Pt 2):43–52.
Liu J, Zhang C, Hu W, Feng Z. Tumor suppressor p53 and metabolism. J Mol Cell Biol. 2019;11(4):284–92.
Munisamy M, Mukherjee N, Thomas L, et al. Therapeutic opportunities in cancer therapy: targeting the p53-MDM2/MDMX interactions. Am J Cancer Res. 2021;11(12):5762–81.
Xia Z, Kon N, Gu AP, Tavana O, Gu W. Deciphering the acetylation code of p53 in transcription regulation and tumor suppression. Oncogene. 2022;41(22):3039–50.
Nagasaka M, Miyajima C, Aoki H, Aoyama M, Morishita D, Inoue Y, Hayashi H. Insights into regulators of p53 acetylation. Cells. 2022;11(23):3825. https://doi.org/10.3390/cells11233825.
Chen S, Seiler J, Santiago-Reichelt M, et al. Repression of RNA polymerase I upon stress is caused by inhibition of RNA-dependent deacetylation of PAF53 by SIRT7. Mol Cell. 2013;52(3):303–13.
Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14(4):dmm047662. https://doi.org/10.1242/dmm.047662.
Carbonell T, Gomes AV. MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 2020;36:101607.
Siebert V, Allencherril J, Ye Y, Wehrens XHT, Birnbaum Y. The role of non-coding RNAs in ischemic myocardial reperfusion injury. Cardiovasc Drugs Ther. 2019;33(4):489–98.
Schmitz SU, Grote P, Herrmann BG. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci. 2016;73(13):2491–509.
Li J, Xuan Z, Liu C. Long non-coding RNAs and complex human diseases. Int J Mol Sci. 2013;14(9):18790–808.
Huang H, Bu YZ, Zhang XY, et al. LINC01433 promotes hepatocellular carcinoma progression via modulating the miR-1301/STAT3 axis. J Cell Physiol. 2019;234(5):6116–24.
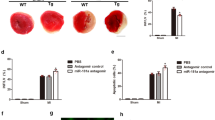
Zhang H, Wang J, Du A, Li Y. MiR-483-3p inhibition ameliorates myocardial ischemia/reperfusion injury by targeting the MDM4/p53 pathway. Mol Immunol. 2020;125:9–14.
Choudhry H, Harris AL. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018;27(2):281–98.
Zheng J, Chen P, Zhong J, Cheng Y, Chen H, He Y, Chen C. HIF-1α in myocardial ischemia-reperfusion injury (Review). Mol Med Rep. 2021;23(5):352. https://doi.org/10.3892/mmr.2021.11991.
Strowitzki MJ, Cummins EP, Taylor CT. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: unique or ubiquitous? Cells. 2019;8(5):384. https://doi.org/10.3390/cells8050384.
Xie L, Pi X, Wang Z, et al. Depletion of PHD3 protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. J Mol Cell Cardiol. 2015;80:156–65.
Jiang M, Jia K, Wang L, et al. Alterations of DNA damage response pathway: Biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B. 2021;11(10):2983–94.
Vadivel Gnanasundram S, Bonczek O, Wang L, Chen S, Fahraeus R. p53 mRNA metabolism links with the DNA damage response. Genes (Basel). 2021;12(9):1446. https://doi.org/10.3390/genes12091446.
Manna PT, Munsey TS, Abuarab N, et al. TRPM2-mediated intracellular Zn2+ release triggers pancreatic beta-cell death. Biochem J. 2015;466(3):537–46.
Colvin RA, Fontaine CP, Laskowski M, Thomas D. Zn2+ transporters and Zn2+ homeostasis in neurons. Eur J Pharmacol. 2003;479(1-3):171–85.
Sikora J, Ouagazzal AM. Synaptic zinc: an emerging player in parkinson’s disease. Int J Mol Sci. 2021;22(9):4724. https://doi.org/10.3390/ijms22094724.
Lin CL, Tseng HC, Chen RF, et al. Intracellular zinc release-activated ERK-dependent GSK-3beta-p53 and Noxa-Mcl-1 signaling are both involved in cardiac ischemic-reperfusion injury. Cell Death Differ. 2011;18(10):1651–63.
Zebrowski DC, Alcendor RR, Kirshenbaum LA, Sadoshima J. Caspase-3 mediated cleavage of MEKK1 promotes p53 transcriptional activity. J Mol Cell Cardiol. 2006;40(5):605–18.
Pei YH, Chen J, Wu X, et al. LncRNA PEAMIR inhibits apoptosis and inflammatory response in PM2.5 exposure aggravated myocardial ischemia/reperfusion injury as a competing endogenous RNA of miR-29b-3p. Nanotoxicology. 2020;14(5):638–53.
Su Q, Lv XW, Xu YL, et al. Exosomal LINC00174 derived from vascular endothelial cells attenuates myocardial I/R injury via p53-mediated autophagy and apoptosis. Mol Ther Nucleic Acids. 2021;23:1304–22.
Guedes EC, da Silva IB, Lima VM, et al. High fat diet reduces the expression of miRNA-29b in heart and increases susceptibility of myocardium to ischemia/reperfusion injury. J Cell Physiol. 2019;234(6):9399–407.
Wang X, Ha T, Zou J, et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102(3):385–95.
Wang S, Cheng Z, Chen X, Xue H. microRNA-135a protects against myocardial ischemia-reperfusion injury in rats by targeting protein tyrosine phosphatase 1B. J Cell Biochem. 2019;120(6):10421–33.
Jiao H, Chen R, Jiang Z, Zhang L, Wang H. miR-22 protect PC12 from ischemia/reperfusion-induced injury by targeting p53 upregulated modulator of apoptosis (PUMA). Bioengineered. 2020;11(1):209–18.
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54(1):146–51.
Angelone T, Filice E, Pasqua T, et al. Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury. Cell Mol Life Sci. 2013;70(3):495–509.
Petzelbauer P, Zacharowski PA, Miyazaki Y, et al. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11(3):298–304.
Ansari J, Kaur G, Gavins FNE. Therapeutic potential of annexin A1 in Ischemia reperfusion injury. Int J Mol Sci. 2018;19(4):1211. https://doi.org/10.3390/ijms19041211.
Yin A, Feng M, Zhang L, et al. Identification of a novel native peptide derived from 60S ribosomal protein L23a that translationally regulates p53 to reduce myocardial ischemia-reperfusion. Pharmacol Res. 2022;175:105988.
Li Y, Meng Q, Wang L, Cui Y. TRIM27 protects against cardiac ischemia-reperfusion injury by suppression of apoptosis and inflammation via negatively regulating p53. Biochem Biophys Res Commun. 2021;557:127–34.
Zhang C, Shi J, Qian L, et al. Nucleostemin exerts anti-apoptotic function via p53 signaling pathway in cardiomyocytes. In Vitro Cell Dev Biol Anim. 2015;51(10):1064–71.
Jiang Y, Yang Y, Zhang Y, et al. Cytoplasmic sequestration of p53 by lncRNA-CIRPILalleviates myocardial ischemia/reperfusion injury. Commun Biol. 2022;5(1):716.
Ka WH, Cho SK, Chun BN, Byun SY, Ahn JC. The ubiquitin ligase COP1 regulates cell cycle and apoptosis by affecting p53 function in human breast cancer cell lines. Breast Cancer. 2018;25(5):529–38.
Moscetti I, Bizzarri AR, Cannistraro S. Imaging and kinetics of the bimolecular complex formed by the tumor suppressor p53 with ubiquitin ligase COP1 as studied by atomic force microscopy and surface plasmon resonance. Int J Nanomed. 2018;13:251–9.
Li X, Ni L, Wang W, Zong L, Yao B. LncRNA Fendrr inhibits hypoxia/reoxygenation-induced cardiomyocyte apoptosis by downregulating p53 expression. J Pharm Pharmacol. 2020;72(9):1211–20.
Zhan LF, Zhang Q, Zhao L, et al. LncRNA-6395 promotes myocardial ischemia-reperfusion injury in mice through increasing p53 pathway. Acta Pharmacol Sin. 2022;43(6):1383–94.
Steenbeke M, De Bruyne S, De Buyzere M, et al. The role of soluble receptor for advanced glycation end-products (sRAGE) in the general population and patients with diabetes mellitus with a focus on renal function and overall outcome. Crit Rev Clin Lab Sci. 2021;58(2):113–30.
Prasad K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol Cell Biochem. 2019;451(1-2):139–44.
Erusalimsky JD. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021;42:101958.
Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79(10):1379–86.
Dang M, Zeng X, Chen B, et al. Interferon-gamma mediates the protective effects of soluble receptor for advanced glycation end-product in myocardial ischemia/reperfusion. Lab Invest. 2019;99(3):358–70.
Calle X, Garrido-Moreno V, Lopez-Gallardo E, et al. Mitochondrial E3 ubiquitin ligase 1 (MUL1) as a novel therapeutic target for diseases associated with mitochondrial dysfunction. IUBMB Life. 2022;74(9):850–65.
Prudent J, Zunino R, Sugiura A, et al. MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol Cell. 2015;59(6):941–55.
Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post-translational modification, SUMOylation, and cancer (Review). Int J Oncol. 2018;52(4):1081–94.
Vertegaal ACO. Signalling mechanisms and cellular functions of SUMO. Nat Rev Mol Cell Biol. 2022;23(11):715–31.
Wang SJ, Chen H, Tang LJ, et al. Upregulation of mitochondrial E3 ubiquitin ligase 1 in rat heart contributes to ischemia/reperfusion injury. Can J Physiol Pharmacol. 2020;98(5):259–66.
Liu X, Xiao J, Zhu H, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21(4):584–95.
Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7(4):433–41.
Zhou Q, Deng J, Yao J, et al. Exercise downregulates HIPK2 and HIPK2 inhibition protects against myocardial infarction. EBioMedicine. 2021;74:103713.
Bauer TM, Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res. 2020;126(2):280–93.
Gan X, Zhang L, Liu B, et al. CypD-mPTP axis regulates mitochondrial functions contributing to osteogenic dysfunction of MC3T3-E1 cells in inflammation. J Physiol Biochem. 2018;74(3):395–402.
Hou D, Hu F, Mao Y, et al. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022;54:102355.
Feng W, Wang J, Li B, et al. Graphene oxide leads to mitochondrial-dependent apoptosis by activating ROS-p53-mPTP pathway in intestinal cells. Int J Biochem Cell Biol. 2022;146:106206.
Yang H, Li R, Zhang L, et al. p53-cyclophilin D mediates renal tubular cell apoptosis in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2019;317(5):F1311–F7.
Zhao J, Liu X, Blayney A, et al. Intrinsically disordered N-terminal domain (NTD) of p53 interacts with mitochondrial PTP regulator cyclophilin D. J Mol Biol. 2022;434(9):167552.
Miura T, Nishihara M, Miki T. Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: role of GSK-3beta in myocardial protection against ischemia/reperfusion injury. J Pharmacol Sci. 2009;109(2):162–7.
Peng K, Liu H, Yan B, et al. Inhibition of cathepsin S attenuates myocardial ischemia/reperfusion injury by suppressing inflammation and apoptosis. J Cell Physiol. 2021;236(2):1309–20.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65.
Palmai-Pallag T, Bachrati CZ. Inflammation-induced DNA damage and damage-induced inflammation: a vicious cycle. Microbes Infect. 2014;16(10):822–32.
Zhou SF, Yuan J, Liao MY, et al. IL-17A promotes ventricular remodeling after myocardial infarction. J Mol Med (Berl). 2014;92(10):1105–16.
Oshima Y, Fujio Y, Nakanishi T, et al. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res. 2005;65(2):428–35.
Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105(6):771–85.
Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109(11):1302–8.
Takahashi J, Yamamoto M, Yasukawa H, et al. Interleukin-22 directly activates myocardial STAT3 (signal transducer and activator of transcription-3) signaling pathway and prevents myocardial ischemia reperfusion injury. J Am Heart Assoc. 2020;9(8):e014814.
Satoh K. Sirtuin-7 as a novel therapeutic target in vascular smooth muscle cell proliferation and remodeling. Circ J. 2021;85(12):2241–2.
Yamamura S, Izumiya Y, Araki S, et al. Cardiomyocyte Sirt (Sirtuin) 7 ameliorates stress-induced cardiac hypertrophy by interacting with and deacetylating GATA4. Hypertension. 2020;75(1):98–108.
Sun M, Zhai M, Zhang N, et al. MicroRNA-148b-3p is involved in regulating hypoxia/reoxygenation-induced injury of cardiomyocytes in vitro through modulating SIRT7/p53 signaling. Chem Biol Interact. 2018;296:211–9.
Paul A, Tang TH, Ng SK. Interferon Regulatory Factor 9 Structure and Regulation. Front Immunol. 2018;9:1831.
Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100.
Jiang DS, Luo YX, Zhang R, et al. Interferon regulatory factor 9 protects against cardiac hypertrophy by targeting myocardin. Hypertension. 2014;63(1):119–27.
Zhang Y, Liu X, She ZG, et al. Interferon regulatory factor 9 is an essential mediator of heart dysfunction and cell death following myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109(5):434.
Agupitan AD, Neeson P, Williams S, Howitt J, Haupt S, Haupt Y. P53: a guardian of immunity becomes its saboteur through mutation. Int J Mol Sci. 2020;21(10):3452. https://doi.org/10.3390/ijms21103452.
Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20(8):471–80.
Yu S, Ji G, Zhang L. The role of p53 in liver fibrosis. Front Pharmacol. 2022;13:1057829.
Zhang W, Gai C, Ding D, Wang F, Li W. Targeted p53 on small-molecules-induced ferroptosis in cancers. Front Oncol. 2018;8:507.
Garbern JC, Lee RT. Mitochondria and metabolic transitions in cardiomyocytes: lessons from development for stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2021;12(1):177.
Marin W, Marin D, Ao X, Liu Y. Mitochondria as a therapeutic target for cardiac ischemia-reperfusion injury (Review). Int J Mol Med. 2021;47(2):485–99.
Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1(4):515–25.
Tan WQ, Wang JX, Lin ZQ, et al. Novel cardiac apoptotic pathway: the dephosphorylation of apoptosis repressor with caspase recruitment domain by calcineurin. Circulation. 2008;118(22):2268–76.
Wang JX, Jiao JQ, Li Q, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17(1):71–8.
Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–95.
Zhang YQ, Herman B. ARC protects rat cardiomyocytes against oxidative stress through inhibition of caspase-2 mediated mitochondrial pathway. J Cell Biochem. 2006;99(2):575–88.
Jo DG, Jun JI, Chang JW, et al. Calcium binding of ARC mediates regulation of caspase 8 and cell death. Mol Cell Biol. 2004;24(22):9763–70.
Foo RS, Chan LK, Kitsis RN, Bennett MR. Ubiquitination and degradation of the anti-apoptotic protein ARC by MDM2. J Biol Chem. 2007;282(8):5529–35.
Yan J, Wan P, Choksi S, Liu ZG. Necroptosis and tumor progression. Trends Cancer. 2022;8(1):21–7.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–21.
Xu T, Ding W, Ao X, et al. ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol. 2019;20:414–26.
Xu D, Zou C, Yuan J. Genetic Regulation of RIPK1 and Necroptosis. Annu Rev Genet. 2021;55:235–63.
Deng XX, Li SS, Sun FY. Necrostatin-1 prevents necroptosis in brains after ischemic stroke via inhibition of RIPK1-mediated RIPK3/MLKL signaling. Aging Dis. 2019;10(4):807–17.
Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20(1):19–33.
Wang K, Liu F, Liu CY, et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23(8):1394–405.
White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6(4):a026120.
Dong Y, Chen H, Gao J, et al. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol. 2019;136:27–41.
Majesky MW. Vascular Development. Arterioscler Thromb Vasc Biol. 2018;38(3):e17–24.
Liu L, Zhao Q, Kong M, et al. Myocardin-related transcription factor A regulates integrin beta 2 transcription to promote macrophage infiltration and cardiac hypertrophy in mice. Cardiovasc Res. 2022;118(3):844–58.
Liu CY, Zhang YH, Li RB, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9(1):29.
Li A, Gao M, Liu B, et al. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022;13(5):444.
Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40(3):e104705.
Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16(7):939–46.
Zhang Y, Liu D, Hu H, et al. HIF-1alpha/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2019;120:109464.
Hoshino A, Matoba S, Iwai-Kanai E, et al. p53-TIGAR axis attenuates mitophagy to exacerbate cardiac damage after ischemia. J Mol Cell Cardiol. 2012;52(1):175–84.
Tang J, Chen L, Qin ZH, Sheng R. Structure, regulation, and biological functions of TIGAR and its role in diseases. Acta Pharmacol Sin. 2021;42(10):1547–55.
Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72.
Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052–9.
Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85.
Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861(8):1893–900.
Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–85.
Feng H, Schorpp K, Jin J, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30(10):3411–23 e7.
Tang LJ, Zhou YJ, Xiong XM, et al. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med. 2021;162:339–52.
Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620.
Liu T, Jiang L, Tavana O, Gu W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79(8):1913–24.
Fang X, Cai Z, Wang H, et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res. 2020;127(4):486–501.
Ma S, Sun L, Wu W, et al. USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Front Physiol. 2020;11:551318.
Xiang M, Lu Y, Xin L, et al. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev. 2021;2021:6614009.
Chen Q, Thompson J, Hu Y, Lesnefsky EJ. The mitochondrial electron transport chain contributes to calpain 1 activation during ischemia-reperfusion. Biochem Biophys Res Commun. 2022;613:127–32.
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278(38):36027–31.
Poynton RA, Hampton MB. Peroxiredoxins as biomarkers of oxidative stress. Biochim Biophys Acta. 2014;1840(2):906–12.
Wang Z, Sun R, Wang G, et al. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020;28:101343.
Chen Q, Thompson J, Hu Y, Lesnefsky EJ. Cardiomyocyte specific deletion of p53 decreases cell injury during ischemia-reperfusion: role of mitochondria. Free Radic Biol Med. 2020;158:162–70.
Cao T, Fan S, Zheng D, et al. Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res Cardiol. 2019;114(3):17.
Chen Q, Thompson J, Hu Y, Dean J, Lesnefsky EJ. Inhibition of the ubiquitous calpains protects complex I activity and enables improved mitophagy in the heart following ischemia-reperfusion. Am J Physiol Cell Physiol. 2019;317(5):C910–C21.
Yu C, Xiao JH. The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid Med Cell Longev. 2021;2021:6635460.
Chen GH, Song CC, Pantopoulos K, et al. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic Biol Med. 2022;180:95–107.
Li H, Zou T, Meng S, Peng YZ, Yang JF. p21 protects cardiomyocytes against ischemia-reperfusion injury by inhibiting oxidative stress. Mol Med Rep. 2018;17(3):4665–71.
Zeng L, Zhang F, Zhang Z, et al. P53 inhibitor pifithrin-alpha inhibits ropivacaine-induced neuronal apoptosis via the mitochondrial apoptosis pathway. J Biochem Mol Toxicol. 2021;35(8):e22822.
Murphy PJ, Galigniana MD, Morishima Y, et al. Pifithrin-alpha inhibits p53 signaling after interaction of the tumor suppressor protein with hsp90 and its nuclear translocation. J Biol Chem. 2004;279(29):30195–201.
Culmsee C, Zhu X, Yu QS, et al. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem. 2001;77(1):220–8.
Liu P, Xu B, Cavalieri TA, Hock CE. Pifithrin-alpha attenuates p53-mediated apoptosis and improves cardiac function in response to myocardial ischemia/reperfusion in aged rats. Shock. 2006;26(6):608–14.
Venkatapuram S, Wang C, Krolikowski JG, et al. Inhibition of apoptotic protein p53 lowers the threshold of isoflurane-induced cardioprotection during early reperfusion in rabbits. Anesth Analg. 2006;103(6):1400–5.
Liu P, Xu B, Cavalieri TA, Hock CE. Inhibition of p53 by pifithrin-alpha reduces myocyte apoptosis and leukocyte transmigration in aged rat hearts following 24 hours of reperfusion. Shock. 2008;30(5):545–51.
Sohn D, Graupner V, Neise D, et al. Pifithrin-alpha protects against DNA damage-induced apoptosis downstream of mitochondria independent of p53. Cell Death Differ. 2009;16(6):869–78.
Zhu J, Singh M, Selivanova G, Peuget S. Pifithrin-alpha alters p53 post-translational modifications pattern and differentially inhibits p53 target genes. Sci Rep. 2020;10(1):1049.
Razvi S, Jabbar A, Pingitore A, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 2018;71(16):1781–96.
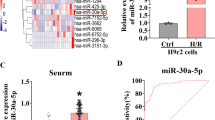
Forini F, Kusmic C, Nicolini G, et al. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology. 2014;155(11):4581–90.
Hsieh SR, Cheng WC, Su YM, Chiu CH, Liou YM. Molecular targets for anti-oxidative protection of green tea polyphenols against myocardial ischemic injury. Biomedicine (Taipei). 2014;4(4):23.
Pervin M, Unno K, Takagaki A, Isemura M, Nakamura Y. Function of green tea catechins in the brain: epigallocatechin gallate and its metabolites. Int J Mol Sci. 2019;20(15):3630. https://doi.org/10.3390/ijms20153630.
Zhang C, Liao P, Liang R, Zheng X, Jian J. Epigallocatechin gallate prevents mitochondrial impairment and cell apoptosis by regulating miR-30a/p53 axis. Phytomedicine. 2019;61:152845.
Chibaya L, Karim B, Zhang H, Jones SN. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc Natl Acad Sci. 2021;118(4):e2003193118. https://doi.org/10.1073/pnas.2003193118.
Sahinovic MM, Struys M, Absalom AR. Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin Pharmacokinet. 2018;57(12):1539–58.
Yan HJ, Qi GQ, Ma Y. Effect of propofol on myocardial ischemia-reperfusion injury through MAPK/ERK pathway. Eur Rev Med Pharmacol Sci. 2019;23(24):11051–61.
Zhao L, Zhuang J, Wang Y, et al. Propofol ameliorates H9c2 cells apoptosis induced by oxygen glucose deprivation and reperfusion injury via inhibiting high levels of mitochondrial fusion and fission. Front Pharmacol. 2019;10:61.
Li S, Lei Z, Yang X, et al. Propofol protects myocardium from ischemia/reperfusion injury by inhibiting ferroptosis through the AKT/p53 signaling pathway. Front Pharmacol. 2022;13:841410.
Wen Z, Mai Z, Zhu X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):36.
Wei L, Zhou Q, Tian H, et al. Integrin beta3 promotes cardiomyocyte proliferation and attenuates hypoxia-induced apoptosis via regulating the PTEN/Akt/mTOR and ERK1/2 pathways. Int J Biol Sci. 2020;16(4):644–54.
Cheng S, Zhang X, Feng Q, et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 2019;227:82–93.
Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. 2011;6(10):e26012.
Gonzalez-Salazar A, Molina-Jijon E, Correa F, et al. Curcumin protects from cardiac reperfusion damage by attenuation of oxidant stress and mitochondrial dysfunction. Cardiovasc Toxicol. 2011;11(4):357–64.
Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019;24(16).
Liu J, Zhu P, Song P, et al. Pretreatment of Adipose Derived Stem Cells with Curcumin Facilitates Myocardial Recovery via Antiapoptosis and Angiogenesis. Stem Cells Int. 2015;2015:638153.
Frump AL, Albrecht M, Yakubov B, Breuils-Bonnet S, Nadeau V, Tremblay E, Potus F, Omura J, Cook T, Fisher A, Rodriguez B, Brown RD, Stenmark KR, Rubinstein CD, Krentz K, Tabima DM, Li R, Sun X, Chesler NC, Provencher S, Bonnet S, Lahm T. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest. 2021;131(6):e129433. https://doi.org/10.1172/jci129433.
da Silva JS, Montagnoli TL, Rocha BS, Tacco MLCA, Marinho SCP, Zapata-Sudo G. Estrogen receptors: therapeutic perspectives for the treatment of Cardiac Dysfunction after Myocardial Infarction. Int J Mol Sci. 2021;22(2):525. https://doi.org/10.3390/ijms22020525.
Patten RD, Pourati I, Aronovitz MJ, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95(7):692–9.
Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281(10):6760–7.
Liu H, Pedram A, Kim JK. Oestrogen prevents cardiomyocyte apoptosis by suppressing p38alpha-mediated activation of p53 and by down-regulating p53 inhibition on p38beta. Cardiovasc Res. 2011;89(1):119–28.
Shlipak MG, Angeja BG, Go AS, et al. Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104(19):2300–4.
Lin Q, Zuo W, Liu Y, Wu K, Liu Q. NAD(+) and cardiovascular diseases. Clin Chim Acta. 2021;515:104–10.
Tannous C, Booz GW, Altara R, et al. Nicotinamide adenine dinucleotide: biosynthesis, consumption and therapeutic role in cardiac diseases. Acta Physiol (Oxf). 2021;231(3):e13551.
Liu L, Wang P, Liu X, et al. Exogenous NAD(+) supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam Clin Pharmacol. 2014;28(2):180–9.
Ramli FF, Ali A, Ibrahim N. Molecular-signaling pathways of ginsenosides Rb in myocardial ischemia-reperfusion injury: a mini review. Int J Med Sci. 2022;19(1):65–73.
Xue Y, Fu W, Liu Y, et al. Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J Food Sci. 2020;85(11):4039–49.
LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev. 2021;42(1):77–96.
Li W, Jin S, Hao J, et al. Metformin attenuates ischemia/reperfusion-induced apoptosis of cardiac cells by downregulation of p53/microRNA-34a via activation of SIRT1. Can J Physiol Pharmacol. 2021;99(9):875–84.
Shanesazzade Z, Peymani M, Ghaedi K, Nasr Esfahani MH. miR-34a/BCL-2 signaling axis contributes to apoptosis in MPP(+) -induced SH-SY5Y cells. Mol Genet Genomic Med. 2018;6(6):975–81.
Yu Y, Zhang X, Li Z, Kong L, Huang Y. LncRNA HOTAIR suppresses TNF-alpha induced apoptosis of nucleus pulposus cells by regulating miR-34a/Bcl-2 axis. Biomed Pharmacother. 2018;107:729–37.
Guo S, Yao Q, Ke Z, et al. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol. 2015;412:85–94.
Tong Z, Xie Y, He M, et al. VDAC1 deacetylation is involved in the protective effects of resveratrol against mitochondria-mediated apoptosis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed Pharmacother. 2017;95:77–83.
De Lazzari F, Prag HA, Gruszczyk AV, Whitworth AJ, Bisaglia M. DJ-1: a promising therapeutic candidate for ischemia-reperfusion injury. Redox Biol. 2021;41:101884.
Huang M, Chen S. DJ-1 in neurodegenerative diseases: pathogenesis and clinical application. Prog Neurobiol. 2021;204:102114.
Xu RY, Xu XW, Deng YZ, et al. Resveratrol attenuates myocardial hypoxia/reoxygenation-induced cell apoptosis through DJ-1-mediated SIRT1-p53 pathway. Biochem Biophys Res Commun. 2019;514(2):401–6.
Takahashi-Niki K, Ganaha Y, Niki T, et al. DJ-1 activates SIRT1 through its direct binding to SIRT1. Biochem Biophys Res Commun. 2016;474(1):131–6.
Zhang Y, Li XR, Zhao L, et al. DJ-1 preserving mitochondrial complex I activity plays a critical role in resveratrol-mediated cardioprotection against hypoxia/reoxygenation-induced oxidative stress. Biomed Pharmacother. 2018;98:545–52.
Zhang X, Zhang Y, Sun A, Ge J. The effects of nicotinamide adenine dinucleotide in cardiovascular diseases: molecular mechanisms, roles and therapeutic potential. Genes Dis. 2022;9(4):959–72.
Hosseini L, Vafaee MS, Mahmoudi J, Badalzadeh R. Nicotinamide adenine dinucleotide emerges as a therapeutic target in aging and ischemic conditions. Biogerontology. 2019;20(4):381–95.
Navas LE, Carnero A. Nicotinamide Adenine Dinucleotide (NAD) Metabolism as a relevant target in cancer. Cells. 2022;11(17):2627. https://doi.org/10.3390/cells11172627.
Arenas-Jal M, Sune-Negre JM, Garcia-Montoya E. Therapeutic potential of nicotinamide adenine dinucleotide (NAD). Eur J Pharmacol. 2020;879:173158.
Funding
This research was supported by grants from the National Natural Science Foundation of China (82202408,81970722 and 82172160).
Author information
Authors and Affiliations
Contributions
Conceptualization, XXZ and ZYX; data collection, curation, and original draft preparation, XXZ and ZQ; review and editing, all authors; visualization, XXZ and ZQ; supervision, SQL and ZYX; project administration and funding acquisition, ZYX. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Xz., Qiu, Z., Lei, Sq. et al. The Role of P53 in Myocardial Ischemia-Reperfusion Injury. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07480-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07480-x