Abstract
Presently, there are many drugs for the treatment of atherosclerosis (AS), among which lipid-lowering, anti-inflammatory, and antiproliferative drugs have been the most studied. These drugs have been shown to have inhibitory effects on the development of AS. Nanoparticles are suitable for AS treatment research due to their fine-tunable and modifiable properties. Compared with drug monotherapy, experimental results have proven that the effects of nanoparticle-encapsulated drugs are significantly enhanced. In addition to nanoparticles containing a single drug, there have been many studies on collaborative drug treatment, collaborative physical treatment (ultrasound, near-infrared lasers, and external magnetic field), and the integration of diagnosis and treatment. This review provides an introduction to the therapeutic effects of nanoparticles loaded with drugs to treat AS and summarizes their advantages, including increased targeting ability, sustained drug release, improved bioavailability, reduced toxicity, and inhibition of plaque and vascular stenosis.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808.
Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115-126.
Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–46.
Xu H, Jiang J, Chen W, et al. Vascular Macrophages in Atherosclerosis. J Immunol Res. 2019;2019:1–14.
Bäck M, Yurdagul A, Tabas I, et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nature Rev Cardiol. 2019;16(7):389–406. https://doi.org/10.1038/s41569-019-0169-2.
Ali AH, Younis N, Abdallah R, et al. Lipid-lowering therapies for atherosclerosis: statins, fibrates, ezetimibe and PCSK9 monoclonal antibodies. Current Med Chem. 2021;28(36):7427–45.
Poznyak AV, Wu W-K, Melnichenko AA, et al. Signaling pathways and key genes involved in regulation of foam cell formation in atherosclerosis. Cells. 2020;9(3):584.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25. https://doi.org/10.1038/nature10146.
Prilepskii AY, Serov NS, Kladko DV, et al. Nanoparticle-based approaches towards the treatment of atherosclerosis. Pharmaceutics. 2020;12(11):1056.
Baetke SC, Lammers T, Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. British J Radiol. 2015;88(1054):20150207.
Flores AM, Ye J, Jarr K-U, et al. Nanoparticle therapy for vascular diseases. Arteriosclerosis, Thrombosis Vasc Biol. 2019;39(4):635–46.
Lobatto ME, Fuster V, Fayad ZA, et al. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discovery. 2011;10(11):835–52. https://doi.org/10.1038/nrd3578.
DiStasio N, Lehoux S, Khademhosseini A, et al. The multifaceted uses and therapeutic advantages of nanoparticles for atherosclerosis research. Materials. 2018;11(5):754.
Behera S, Pramanik K, Nayak M. Recent advancement in the treatment of cardiovascular diseases: conventional therapy to nanotechnology. Current Pharmaceut Design. 2015;21(30):4479–97.
Shen Y, Liang L, Zhang S, et al. Organelle-targeting surface-enhanced Raman scattering (SERS) nanosensors for subcellular pH sensing. Nanoscale. 2018;10(4):1622–30.
Leal BH, Velasco B, Cambón A, et al. Combined therapeutics for atherosclerosis treatment using polymeric nanovectors. Pharmaceutics. 2022;14(2):258.
Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622. https://doi.org/10.1038/s41467-020-16439-7.
Jiang C, Qi Z, Tang Y, et al. Rational design of lovastatin-loaded spherical reconstituted high density lipoprotein for efficient and safe anti-atherosclerotic therapy. Mol Pharmaceut. 2019;16(7):3284–91.
Wei B, Li Y, Ao M, et al. Ganglioside GM3-functionalized reconstituted high-density lipoprotein (GM3-rHDL) as a novel nanocarrier enhances antiatherosclerotic efficacy of statins in apoE−/− C57BL/6 Mice. Pharmaceutics. 2022;14(11):2534.
Jiang C, Qi Z, He W, et al. Dynamically enhancing plaque targeting via a positive feedback loop using multifunctional biomimetic nanoparticles for plaque regression. J Controlled Release. 2019;308:71–85.
Sun Y, Chen L, Zhao S, et al. Effects of nanoparticle-mediated delivery of pitavastatin on atherosclerotic plaques in ApoE-knockout mice and THP-1-derived macrophages. Exp Ther Med. 2020;19(6):3787–97.
Rakshit M, Darwitan A, Muktabar A, et al. Anti-inflammatory potential of simvastatin loaded nanoliposomes in 2D and 3D foam cell models. Nanomed: Nanotechnol, Biol Med. 2021;37:102434.
Imanparast F, Faramarzi MA, Vatannejad A, et al. mZD7349 peptide-conjugated PLGA nanoparticles directed against VCAM-1 for targeted delivery of simvastatin to restore dysfunctional HUVECs. Microvasc Res. 2017;112:14–9.
Pham LM, Kim E-C, Ou W, et al. Targeting and clearance of senescent foamy macrophages and senescent endothelial cells by antibody-functionalized mesoporous silica nanoparticles for alleviating aorta atherosclerosis. Biomaterials. 2021;269:120677.
Chen L, Wang C, Wu Y. Cholesterol (Blood lipid) lowering potential of Rosuvastatin chitosan nanoparticles for atherosclerosis: preclinical study in rabbit model. Acta Biochim Pol. 2020;67(4):495–9.
Saraogi GK, Tholiya S, Mishra Y, et al. Formulation development and evaluation of pravastatin-loaded nanogel for hyperlipidemia management. Gels. 2022;8(2):81.
du Toit LC, Hulisani Demana P, Essop CY. A nano-enabled biotinylated anti-LDL theranostic system to modulate systemic LDL cholesterol. Int J Pharmaceut. 2022;628:122258.
Chen F, Chen J, Han C, et al. Theranostics of atherosclerosis by the indole molecule-templated self-assembly of probucol nanoparticles. J Mater Chem B. 2021;9(20):4134–42.
Liang X, Li H, Zhang A, et al. Red blood cell biomimetic nanoparticle with anti-inflammatory, anti-oxidative and hypolipidemia effect ameliorated atherosclerosis therapy. Nanomed: Nanotechnol Biol Med. 2022;41:102519.
Chen L, Yang J, Fu X, et al. A targeting mesoporous dopamine nanodrug platform with NIR responsiveness for atherosclerosis improvement. Biomater Adv. 2022;136:212775.
Fu X, Yu X, Jiang J, et al. Small molecule-assisted assembly of multifunctional ceria nanozymes for synergistic treatment of atherosclerosis. Nat Commun. 2022;13(1):6528. https://doi.org/10.1038/s41467-022-34248-y.
Bulgarelli A, Martins Dias AA, Caramelli B, et al. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59(4):308–14. https://doi.org/10.1097/FJC.0b013e318241c385.
You S, Guo X, Xue X, et al. PCSK9 Hapten multicopy displayed onto carrier protein nanoparticle: an antiatherosclerosis vaccine. ACS Biomater Sci Eng. 2019;5(9):4263–71.
Satny M, Hubacek JA, Vrablik M. Statins and inflammation. Curr Atheroscler Rep. 2021;23(12):80. https://doi.org/10.1007/s11883-021-00977-6.
Valanti E, Tsompanidis A, Sanoudou D. Pharmacogenomics in the development and characterization of atheroprotective drugs. Methods Mol Biol. 2014;1175:259–300. https://doi.org/10.1007/978-1-4939-0956-8_11.
Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5(1):3065. https://doi.org/10.1038/ncomms4065.
Koga J-i, Matoba T, Egashira K. Anti-inflammatory nanoparticle for prevention of atherosclerotic vascular diseases. J Atherosclerosis Thrombosis. 2016;23(7):757–65.
Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80(1):106–7.
Laufs U, Scharnagl H, März W. Statin intolerance. Curr Opin Lipidol. 2015;26(6):492–501.
Nenna A, Nappi F, Larobina D, et al. Polymers and nanoparticles for statin delivery: current use and future perspectives in cardiovascular disease. Polymers. 2021;13(5):711.
Hägg D, Sjöberg S, Hultén LM, et al. Augmented levels of CD44 in macrophages from atherosclerotic subjects: a possible IL-6–CD44 feedback loop? Atherosclerosis. 2007;190(2):291–7.
Jain M, He Q, Lee WS, et al. Role of CD44 in the reaction of vascular smooth muscle cells to arterial wall injury. J Clin Investig. 1996;97(3):596–603.
Cuff CA, Kothapalli D, Azonobi I, et al. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Investig. 2001;108(7):1031–40.
McKee CM, Penno MB, Cowman M, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Investig. 1996;98(10):2403–13.
Kanwar RK, Chaudhary R, Tsuzuki T, et al. Emerging engineered magnetic nanoparticulate probes for targeted MRI of atherosclerotic plaque macrophages. Nanomedicine. 2012;7(5):735–49.
Zhao Y, Jiang C, He J, et al. Multifunctional dextran sulfate-coated reconstituted high density lipoproteins target macrophages and promote beneficial antiatherosclerotic mechanisms. Bioconjugate Chem. 2017;28(2):438–48.
Andrews J, Janssan A, Nguyen T, et al. Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: rationale and design of the CARAT study. Cardiovasc Diagnosis Ther. 2017;7(1):45–51.
Huang J, Wang D, Huang L-H, et al. Roles of reconstituted high-density lipoprotein nanoparticles in cardiovascular disease: a new paradigm for drug discovery. Int J Mol Sci. 2020;21(3):739.
Park K, Skidmore S, Hadar J, et al. Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. JControlled Release. 2019;304:125–34.
Chen L, Nakano K, Kimura S, et al. Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension. 2011;57(2):343–50.
Jafari S, Derakhshankhah H, Alaei L, et al. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed Pharmacother. 2019;109:1100–11.
Nishida M. Miyagawa J-i, Yamashita S et al. Nishida M, Miyagawa J, Yamashita S, et al. Localization of CD9, an enhancer protein for proheparin-binding epidermal growth factor-like growth factor, in human atherosclerotic plaques: possible involvement of juxtacrine growth mechanism on smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2000;20(5):1236–43.
Rizeq BR, Younes NN, Rasool K, et al. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int J Mol Sci. 2019;20(22):5776.
Aguiar C, Alegria E, Bonadonna RC, et al. A review of the evidence on reducing macrovascular risk in patients with atherogenic dyslipidaemia: a report from an expert consensus meeting on the role of fenofibrate–statin combination therapy. Atherosclerosis Suppl. 2015;19:1–12.
Guo X, Wang L, Xia X, et al. Effects of atorvastatin and/or probucol on recovery of atherosclerosis in high-fat-diet-fed apolipoprotein E–deficient mice. Biomed Pharmacother. 2019;109:1445–53.
Daugherty A, Zweifel BS, Schonfeld G. The effects of probucol on the progression of atherosclerosis in mature Watanabe heritable hyperlipidaemic rabbits. British J Pharmacol. 1991;103(1):1013–8.
Emlen W, Carl V, Burdick G. Mechanism of transfer of immune complexes from red blood cell CR1 to monocytes. Clin Exp Immunol. 2008;89(1):8–17.
Luk BT, Jack Hu C-M, Fang RH, et al. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale. 2013;6(5):2730–7.
Xing Y, Zhang J, Chen F, et al. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale. 2017;9(25):8781–90.
Li J, Duan H, Pu K. Nanotransducers for Near-Infrared Photoregulation in Biomedicine. Adv Mater. 2019;31(33):1901607.
Momtazi-Borojeni AA, Jaafari MR, Afshar M, et al. PCSK9 immunization using nanoliposomes: preventive efficacy against hypercholesterolemia and atherosclerosis. Arch Med Sci. 2021;17(5):1365–77.
Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Accounts Chem Res. 2011;44(10):1050–60.
Di Mascolo D, Lyon CJ, Aryal S, et al. Rosiglitazone-loaded nanospheres for modulating macrophage-specific inflammation in obesity. J Controlled Release. 2013;170(3):460–8.
Di Francesco V, Gurgone D, Palomba R, et al. Modulating lipoprotein transcellular transport and atherosclerotic plaque formation in ApoE-/- mice via nanoformulated lipid-methotrexate conjugates. ACS Appl Mater Interfaces. 2020;12(34):37943–56.
Bulgarelli A, Leite ACA, Dias AAM, et al. Anti-atherogenic effects of methotrexate carried by a lipid nanoemulsion that binds to LDL receptors in cholesterol-fed rabbits. Cardiovasc Drugs Ther. 2013;27(6):531–9. https://doi.org/10.1007/s10557-013-6488-3.
Gomes FLT, Maranhão RC, Tavares ER, et al. Regression of atherosclerotic plaques of cholesterol-fed rabbits by combined chemotherapy with paclitaxel and methotrexate carried in lipid core nanoparticles. J Cardiovasc Pharmacol Therapeut. 2018;23(6):561–9.
He P, Tang B, Li Y, et al. Effective oxidation-responsive polyester nanocarriers for anti-inflammatory drug delivery. Int J Nanomed. 2021;16:5053–64.
Sun W, Xu Y, Yao Y, et al. Self-oxygenation mesoporous MnO2 nanoparticles with ultra-high drug loading capacity for targeted arteriosclerosis therapy. J Nanobiotechnol. 2022;20(1):88.
Hou X, Lin H, Zhou X, et al. Novel dual ROS-sensitive and CD44 receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis. Carbohydrate Polymers. 2020;232:115787.
Meng N, Gong Y, Zhang J, et al. A novel curcumin-loaded nanoparticle restricts atherosclerosis development and promotes plaques stability in apolipoprotein E deficient mice. J Biomater Appl. 2019;33(7):946–54.
Pillai SC, Borah A, Le MNT, et al. Co-delivery of curcumin and bioperine via PLGA nanoparticles to prevent atherosclerotic foam cell formation. Pharmaceutics. 2021;13(9):1420. https://doi.org/10.3390/pharmaceutics13091420.
J.B VK, Ramakrishna S, Madhusudhan B. Preparation and characterisation of atorvastatin and curcumin-loaded chitosan nanoformulations for oral delivery in atherosclerosis. IET Nanobiotechnol. 2017;11(1):96–103.
Qiu P, Xu Y. The construction of multifunctional nanoparticles system for dual-modal imaging and arteriosclerosis targeted therapy. Am J Transl Res. 2021;13(5):4026–39.
Sherin S, Balachandran S, Abraham A. Curcumin incorporated titanium dioxide nanoparticles as MRI contrasting agent for early diagnosis of atherosclerosis- rat model. Vet Animal Sci. 2020;10:100090.
Matuszak J, Lutz B, Sekita A, et al. Drug delivery to atherosclerotic plaques using superparamagnetic iron oxide nanoparticles. Int J Nanomed. 2018;13:8443–60.
Kheirolomoom A, Dayton PA, Lum AFH, et al. Acoustically-active microbubbles conjugated to liposomes: characterization of a proposed drug delivery vehicle. J Controlled Release. 2007;118(3):275–84.
Luo X, Fu H, Xu C, et al. Efficient treatment of atherosclerosis by dexamethasone acetate and rapamycin Co-loaded mPEG-DSPE calcium phosphate nanoparticles. J Biomed Nanotechnol. 2020;16(6):810–26.
Hou C, Bai H, Wang Z, et al. A hyaluronan-based nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohydrate Polymers. 2018;195:339–48.
Coomes E, Chan ESL, Reiss AB. Methotrexate in atherogenesis and cholesterol metabolism. Cholesterol. 2011;2011:1–8.
Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13(11):1109–13.
Chen AJC, Su, et al. Folate receptor-targeting and reactive oxygen species-responsive liposomal formulation of methotrexate for treatment of rheumatoid arthritis. Pharmaceutics. 2019;11(11):582.
Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467–75.
Hirata RDC, Hirata MH, Mesquita CH, et al. E¡ects of apolipoprotein B-100 on the metabolism of a lipid microemulsion model in rats. Biochim Biophys Acta. 1999;1437(1):53–62.
Maranhão RC, Roland IA, Toffoletto O, et al. Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors. Lipids. 1997;32(6):627–33.
Boada CA, Zinger A, Rohen S, et al. LDL-based lipid nanoparticle derived for blood plasma accumulates preferentially in atherosclerotic plaque. Front Bioeng Biotechnol. 2021;9:794676.
Campbell MS, Fleenor BS. The emerging role of curcumin for improving vascular dysfunction: a review. Crit Rev Food Sci Nutrition. 2018;58(16):2790–9.
Li X, Fang Q, Tian X, et al. Curcumin attenuates the development of thoracic aortic aneurysm by inhibiting VEGF expression and inflammation. Mol Med Reports. 2017;16(4):4455–62.
Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94.
Giuliani C. The Flavonoid Quercetin Induces AP-1 Activation in FRTL-5 Thyroid Cells. Antioxidants. 2019;8(5):112.
Gupta SC, Patchva S, Koh W, et al. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–99.
Flora G, Gupta D, Tiwari A. Nanocurcumin: A Promising Therapeutic Advancement over Native Curcumin. Crit Rev Therapeutic Drug Carrier Syst. 2013;30(4):331–68.
Wang Y-J, Pan M-H, Cheng A-L, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharmaceut Biomed Anal. 1997;15(12):1867–76.
Allijn IE, Schiffelers RM, Storm G. Comparison of pharmaceutical nanoformulations for curcumin: Enhancement of aqueous solubility and carrier retention. Int J Pharmaceut. 2016;506(1-2):407–13.
Poon IKH, Lucas CD, Rossi AG, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–80. https://doi.org/10.1038/nri3607.
Ravichandran KS. Beginnings of a Good Apoptotic Meal: The Find-Me and Eat-Me Signaling Pathways. Immunity. 2011;35(4):445–55.
Bagalkot V, Deiuliis JA, Rajagopalan S, et al. “Eat me” imaging and therapy. Adv Drug Delivery Rev. 2016;99:2–11.
Shi D, Fu M, Fan P, et al. Artificial phosphatidylserine liposome mimics apoptotic cells in inhibiting maturation and immunostimulatory function of murine myeloid dendritic cells in response to 1-chloro-2,4-dinitrobenze in vitro. Arch Dermatol Res. 2007;299(7):327–36. https://doi.org/10.1007/s00403-007-0770-9.
Wang J, Kang Y-X, Pan W, et al. Enhancement of anti-inflammatory activity of curcumin using phosphatidylserine-containing nanoparticles in cultured macrophages. Int J Mol Sci. 2016;17(6):969.
Randhawa G, Kullar J, Rajkumar. Bioenhancers from mother nature and their applicability in modern medicine. Int J Appl Basic Med Res. 2011;1(1):5–10.
Zheng S, Zhang M, Bai H, et al. Preparation of AS1411 aptamer modified Mn-MoS2 QDs for targeted MR imaging and fluorescence labelling of renal cell carcinoma. Int J Nanomed. 2019;14:9513–24.
Fan X, Yuan Z, Shou C, et al. cRGD-Conjugated Fe2O4@PDA-DOX Multifunctional Nanocomposites for MRI and Antitumor Chemo-Photothermal Therapy. Int J Nanomed. 2019;14:9631–45.
Luscinskas FW, Gerszten RE, Garcia-Zepeda EA, et al. C-C and C-X-C Chemokines Trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Annals New York Acad Sci. 2006;902(1):288–93.
Cushing SD, Berliner JA, Valente AJ, et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci. 1990;87(13):5134–8.
Minnekhanov AA, Deygen DM, Konstantinova EA, et al. Paramagnetic properties of carbon-doped titanium dioxide. Nanoscale Res Lett. 2012;7(1):333.
Poon M, Gertz SD, Fallon JT, et al. Dexamethasone inhibits macrophage accumulation after balloon arterial injury in cholesterol fed rabbits. Atherosclerosis. 2001;155(2):371–80.
Li Z, Huang H, Huang L, et al. Prevention of oxidized low density lipoprotein-induced endothelial cell injury by DA-PLGA-PEG-cRGD nanoparticles combined with ultrasound. Int J Mol Sci. 2017;18(4):815.
Pakala R, Stabile E, Jang GJ, et al. Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J Cardiovasc Pharmacol. 2005;46(4):481–6.
Wang Y, Zhang K, Li T, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–80.
Wang Y, Zhang K, Qin X, et al. Biomimetic nanotherapies: red blood cell based core–shell structured nanocomplexes for atherosclerosis management. Adv Sci. 2019;6(12):1900172.
Song Y, Huang Z, Liu X, et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice. Nanomed: Nanotechnol Biol Med. 2019;15(1):13–24.
Huang C, Huang W, Zhang L, et al. Targeting peptide, fluorescent reagent modified magnetic liposomes coated with Rapamycin target early atherosclerotic plaque and therapy. Pharmaceutics. 2022;14(5):1083.
Dou Y, Guo J, Chen Y, et al. Sustained delivery by a cyclodextrin material-based nanocarrier potentiates antiatherosclerotic activity of rapamycin via selectively inhibiting mTORC1 in mice. J Controlled Release. 2016;235:48–62.
Zhou J, Niu C, Huang B, et al. Platelet membrane biomimetic nanoparticles combined with UTMD to improve the stability of atherosclerotic plaques. Front Chem. 2022;10:868063.
Cheraga N, Ye Z, Xu M-J, et al. Targeted therapy of atherosclerosis by pH-sensitive hyaluronic acid nanoparticles co-delivering all-trans retinal and rapamycin. Nanoscale. 2022;14(24):8709–26.
Wang Q, Duan Y, Jing H, et al. Inhibition of atherosclerosis progression by modular micelles. J Controlled Release. 2023;354:294–304.
Zhu X, Xie H, Liang X, et al. Bilayered nanoparticles with sequential release of VEGF gene and paclitaxel for restenosis inhibition in atherosclerosis. ACS Appl Mater Interfaces. 2017;9(33):27522–32.
Shiozaki AA, Senra T, Morikawa AT, et al. Treatment of patients with aortic atherosclerotic disease with paclitaxel-associated lipid nanoparticles. Clinics. 2016;71(8):435–9.
Haeri A, Osouli M, Bayat F, et al. Nanomedicine approaches for sirolimus delivery: a review of pharmaceutical properties and preclinical studies. Artificial Cells, Nanomed Biotechnol. 2018;46(sup1):1–14.
Martinet W, De Loof H, De Meyer GRY. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis. 2014;233(2):601–7.
Xuan M, Shao J, Dai L, et al. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–8.
Cao H, Dan Z, He X, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–48.
Hu C-MJ, Zhang L, Aryal S, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci. 2011;108(27):10980–5.
Boada C, Zinger A, Tsao C, et al. Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circulat Res. 2020;126(1):25–37.
Sun W, Li Z, Zhou X, et al. Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Delivery. 2019;26(1):45–50.
Bekeredjian R, Chen S, Frenkel PA, et al. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108(8):1022–6.
Craik DJ, Fairlie DP, Liras S, et al. The Future of Peptide-based Drugs. Chem Biol Drug Design. 2013;81(1):136–47.
Long Y, Lu Z, Xu S, et al. Self-delivery micellar nanoparticles prevent premetastatic niche formation by interfering with the early recruitment and vascular destruction of granulocytic myeloid-derived suppressor cells. Nano Lett. 2020;20(4):2219–29.
Ng VG, Mena C, Pietras C, et al. Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur J Clin Investig. 2015;45(3):333–45.
Sun D, Zheng Y, Yin T, et al. Coronary drug-eluting stents: from design optimization to newer strategies: coronary Drug-Eluting Stents. J Biomed Mater Res Part A. 2014;102(5):1625–40.
Wiskirchen J, Schöber W, Schart N, et al. The effects of paclitaxel on the three phases of restenosis: smooth muscle cell proliferation, migration, and matrix formation: an in vitro study. Investig Radiol. 2004;39(9):565–71. https://doi.org/10.1097/01.rli.0000133815.22434.55.
Hong MK, Kornowski R, Bramwell O, et al. Paclitaxel-coated GianturcoRoubin II (GR II) stents reduce neointimal hyperplasia in a porcine coronary in-stent restenosis model. Coron Artery Dis. 2001;12(6):513–5. https://doi.org/10.1097/00019501-200109000-00011.
de la Llera-Moya M, Rothblat GH, Glick JM, et al. Etoposide treatment suppresses atherosclerotic plaque development in cholesterol-fed rabbits. Arterioscler Thromb. 1992;12(11):1363–70.
Westedt U, Kalinowski M, Wittmar M, et al. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J Controlled Release. 2007;119(1):41–51.
Bietenbeck M, Florian A, Faber C, et al. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: where are we now? Int J Nanomed. 2016;11:3191–203. https://doi.org/10.2147/IJN.S110542.
Olie RH, van der Meijden PEJ, Spronk HMH, et al. Antithrombotic therapy: prevention and treatment of atherosclerosis and atherothrombosis. In: von Eckardstein A, Binder CJ (eds). Prevention and treatment of atherosclerosis: improving state-of-the-art management and search for novel targets. Cham (CH): Springer; 2020. pp. 103–130. https://doi.org/10.1007/164_2020_357.
Tu S, He W, Han J, et al. Advances in imaging and treatment of atherosclerosis based on organic nanoparticles. APL Bioeng. 2022;6(4):041501.
Dai T, He W, Tu S, et al. Black TiO2 nanoprobe-mediated mild phototherapy reduces intracellular lipid levels in atherosclerotic foam cells via cholesterol regulation pathways instead of apoptosis. Bioactive Mater. 2022;17:18–28.
Han XB, Li HX, Jiang YQ, et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017;8(6):e2864. https://doi.org/10.1038/cddis.2017.242.
Zhu X, Wang H, Zheng L, et al. Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway. Int J Nanomed. 2015;10:3719–36. https://doi.org/10.2147/IJN.S82162.
Han X, Kou J, Zheng Y, et al. ROS generated by upconversion nanoparticle-mediated photodynamic therapy induces autophagy via PI3K/AKT/ mTOR signaling pathway in M1 peritoneal macrophage. Cell Physiol Biochem. 2019;52(6):1325–38.
Mu D, Wang X, Wang H, et al. Chemiexcited photodynamic therapy integrated in polymeric nanoparticles capable of MRI against atherosclerosis. Int J Nanomed. 2022;17:2353–66.
Acknowledgments
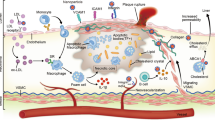
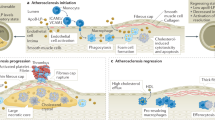
Figure 1 was drawn by Figdraw (https://www.figdraw.com), export ID ASYIT0f1e3. The materials contained in the image are copyrighted by Home for Researchers.
Funding
This work was supported by the Shanxi Provincial Department of Science and Technology (Grant numbers 202103021224003 and 20210302124402), Regional Medical Center Science and Technology Innovation program project (Grant numbers 202206 and 202256), and Taiyuan Municipal Health Commission Project (Grant numbers Z2022001).
Author information
Authors and Affiliations
Contributions
This review was designed by Y.M. This manuscript was written by T.S and K.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, T., Liu, K., Peng, Y. et al. Research progress on the therapeutic effects of nanoparticles loaded with drugs against atherosclerosis. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07461-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07461-0