Abstract
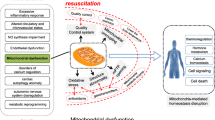
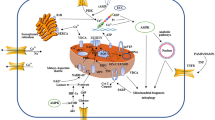
Sepsis is an increasingly worldwide problem; it is currently regarded as a complex life-threatening dysfunction of one or more organs as a result of dysregulated host immune response to infections. The heart is one of the most affected organs, as roughly 10% to 70% of sepsis cases are estimated to turn into sepsis-induced cardiomyopathy (SIC). SIC can be defined as a reversible myocardial dysfunction characterized by dilated ventricles, impaired contractility, and decreased ejection fraction. Mitochondria play a critical role in the normal functioning of cardiac tissues as the heart is highly dependent on its production of adenosine triphosphate (ATP), its damage during SIC includes morphology impairment, mitophagy, biogenesis disequilibrium, electron transport chain disturbance, molecular damage from the actions of pro-inflammatory cytokines and many other different impairments that are major contributing factors to the severity of SIC. Although mitochondria-targeted therapies usage is still inadequate in clinical settings, the preclinical study outcomes promise that the implementation of these therapies may effectively treat SIC. This review summarizes the different therapeutic strategies targeting mitochondria structure, quality, and quantity abnormalities for the treatment of SIC.

Similar content being viewed by others
Data Availability
The authors confirm that the data used to support the findings of this article are included within the article.
Code Availability
Not applicable.
Abbreviations
- AIF:
-
Apoptosis-inducing factor
- AMPKα:
-
Adenosine monophosphate-activated protein kinase α
- ATP:
-
Adenosine triphosphate
- BCL-2:
-
B cell lymphoma-2
- BCL2-L-13:
-
BCL2-like-13
- BNIP3:
-
BCL2/adenovirus E1B 19 kDa interacting protein 3
- CASP1:
-
Caspase-1
- CAT:
-
Catalase
- cAMP:
-
Cyclic adenosine monophosphate
- CLP:
-
Cecal ligation and puncture
- CoQ10:
-
Coenzyme Q10
- CORM-2:
-
CO-releasing molecules-2
- cTnT:
-
Cardiac troponin
- DAMPs:
-
Damage-associated molecular patterns
- DCM:
-
Dilated cardiomyopathy
- DRP:
-
Dynamin-related protein
- eNOS:
-
Endothelial nitric oxide synthase
- ERR:
-
Estrogen-related receptor
- ETC:
-
Electron transport chain
- FNDC5:
-
Fibronectin type III domain containing 5
- FUNDC1:
-
FUN14 domain-containing protein 1
- GSDMD:
-
Gasdermin D
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- Gαq:
-
Gq protein alpha subunit
- HF:
-
Heart failure
- huMSCs:
-
Human umbilical cord mesenchymal stem cells
- IL:
-
Interleukin
- IHD:
-
Ischemic heart disease
- iNOS:
-
Inducible nitric oxide synthase
- IMM:
-
Inner mitochondria membrane
- JNK-LATS2:
-
Jun N-terminal kinase-large tumor suppressor 2
- LC3:
-
Light chain 3
- LTCC:
-
L-type calcium channel
- LPS:
-
Lipopolysaccharide
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular fraction shortening
- MAPK:
-
Mitogen-activated protein kinase
- MitoQ:
-
Mitochondria-targeted ubiquinone
- mPTP:
-
Mitochondrial permeability transition pore
- MFF:
-
Mitochondrial fission factor
- Mfn:
-
Mitofusin
- mTOR:
-
Mammalian target of rapamycin
- mtDNA:
-
Mitochondria DNA
- MUL1:
-
Mitochondrial ubiquitin ligase 1
- NAD:
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NLRP3:
-
Nucleotide-binding domain leucine-rich repeat, pyrin domain–containing-3
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NOX2:
-
NADPH oxidase-2
- NaHS:
-
Sodium hydrosulfide
- NF-κB:
-
Nuclear factor kappa B
- NRF:
-
Nuclear respiratory factor
- OCR:
-
Oxygen consumption rate
- OMM:
-
Outer mitochondria membrane
- OPA1:
-
Optic atrophy protein 1
- OXPHOS:
-
Oxidative phosphorylation
- PDC:
-
Pyruvate dehydrogenase complex
- PDK:
-
Pyruvate dehydrogenase kinase
- PEG-SOD:
-
Polyethylene glycol-conjugated–superoxide dismutase
- PGAM5:
-
Phosphoglycerate mutase family member 5
- PHB:
-
Prohibitin
- PINK1:
-
PTEN-induced putative protein kinase 1
- PI3K:
-
Phosphatidylinositol 3-kinases
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ coactivator-1α
- PPAR:
-
Peroxisome proliferated-activated receptor
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- PTEN:
-
Phosphatase and tensin homolog
- ROS:
-
Reactive oxygen species
- RyR:
-
Ryanodine receptor
- SESN2:
-
Sestrin 2
- SERCA:
-
Sarcoplasmic endoplasmic reticulum Ca2+-ATPase
- SHP:
-
Small heterodimer partner
- SIC:
-
Sepsis-induced cardiomyopathy
- SIMD:
-
Sepsis-induced myocardial dysfunction
- SIRT:
-
Sirtuin
- SOD:
-
Superoxide dismutase
- SUMO:
-
Small ubiquitin-like modifier
- TCA:
-
Tricarboxylic acid
- TLR7:
-
Toll-like receptor 7
- TFAM:
-
Mitochondrial transcription factor A
- TNF- α:
-
Tumor necrosis factor-α
- UCP:
-
Uncoupling protein
References
Wasyluk W, Nowicka-Stążka P, Zwolak A. Heart Metabolism in Sepsis-Induced Cardiomyopathy—Unusual Metabolic Dysfunction of the Heart. Int J Environ Res Public Health. 2021;18(14):7598.
Donnino MW, Andersen LW, Chase M, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. 2016;44(2):360.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Walley KR. Sepsis-induced myocardial dysfunction. Curr Opin Crit Care. 2018;24(4):292–9.
Beesley SJ, Weber G, Sarge T, et al. Septic cardiomyopathy. Crit Care Med. 2018;46(4):625–34.
Rackow E, Kaufman B, Falk J, Astiz M, Weil M. Hemodynamic response to fluid repletion in patients with septic shock: evidence for early depression of cardiac performance. Circ Shock. 1987;22(1):11–22.
Martin L, Derwall M, Al Zoubi S, et al. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest. 2019;155(2):427–37.
Tsolaki V, Makris D, Mantzarlis K, Zakynthinos E. Sepsis-induced cardiomyopathy: oxidative implications in the initiation and resolution of the damage. Oxidative Medicine and Cellular Longevity. 2017;2017.
Pan P, Wang X, Liu D. The potential mechanism of mitochondrial dysfunction in septic cardiomyopathy. J Int Med Res. 2018;46(6):2157–69.
Jarczak D, Kluge S, Nierhaus A. Sepsis-Pathophysiology and Therapeutic Concepts. Front Med (Lausanne). 2021;8:628302.
Zang Q, Maass DL, Tsai SJ, Horton JW. Cardiac mitochondrial damage and inflammation responses in sepsis. Surg Infect. 2007;8(1):41–54.
Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424–34.
Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK. Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study. Medicine (Baltimore). 2016;95(39):e5031.
Jeong HS, Lee TH, Bang CH, Kim JH, Hong SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine (Baltimore). 2018;97(13):e0263.
L’Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. Sepsis-Induced Cardiomyopathy: a Comprehensive Review. Curr Cardiol Rep. 2020;22(5):35.
Mekontso Dessap A, Razazi K, Brun-Buisson C, Deux J-F. Myocardial viability in human septic heart. Intensive Care Med. 2014;40(11):1746–8.
Charpentier J, Luyt C-E, Fulla Y, et al. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32(3):660–5.
Mehta NJ, Khan IA, Gupta V, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95(1):13–7.
Werdan K, Oelke A, Hettwer S, et al. Septic cardiomyopathy: hemodynamic quantification, occurrence, and prognostic implications. Clin Res Cardiol. 2011;100(8):661–8.
Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3(1):1–7.
Zangrillo A, Putzu A, Monaco F, et al. Levosimendan reduces mortality in patients with severe sepsis and septic shock: a meta-analysis of randomized trials. J Crit Care. 2015;30(5):908–13.
Schlesinger JJ, Burger CF. Methylene Blue for Acute Septic Cardiomyopathy in a Burned Patient. J Burn Care Res. 2016;37(3):e287–91.
Fan Y, Jiang M, Gong D, Zou C. Efficacy and safety of low-molecular-weight heparin in patients with sepsis: a meta-analysis of randomized controlled trials. Sci Rep. 2016;6:25984.
Krishnan K, Wassermann TB, Tednes P, Bonderski V, Rech MA. Beyond the bundle: Clinical controversies in the management of sepsis in emergency medicine patients. Am J Emerg Med. 2022;51:296–303.
Vogel DJ, Murray J, Czapran AZ, et al. Veno-arterio-venous ECMO for septic cardiomyopathy: a single-centre experience. Perfusion. 2018;33(1_suppl):57–64.
Alvarez S, Vico T, Vanasco V. Cardiac dysfunction, mitochondrial architecture, energy production, and inflammatory pathways: Interrelated aspects in endotoxemia and sepsis. Int J Biochem Cell Biol. 2016;81:307–14.
Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84(2–3):153–66.
Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–6.
Bernardi P, Krauskopf A, Basso E, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273(10):2077–99.
Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462(2):245–53.
Larche J, Lancel S, Hassoun SM, et al. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J Am Coll Cardiol. 2006;48(2):377–85.
Pan P, Zhang H, Su L, Wang X, Liu D. Melatonin balance the autophagy and apoptosis by regulating UCP2 in the LPS-induced cardiomyopathy. Molecules. 2018;23(3):675.
Fauvel H, Marchetti P, Obert G, et al. Protective effects of cyclosporin A from endotoxin-induced myocardial dysfunction and apoptosis in rats. Am J Respir Crit Care Med. 2002;165(4):449–55.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359.
Hu Y, Yan JB, Zheng MZ, et al. Mitochondrial aldehyde dehydrogenase activity protects against lipopolysaccharide-induced cardiac dysfunction in rats. Mol Med Rep. 2015;11(2):1509–15.
MacGarvey NC, Suliman HB, Bartz RR, et al. Activation of mitochondrial biogenesis by heme oxygenase-1–mediated NF-E2–related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185(8):851–61.
Durand A, Duburcq T, Dekeyser T, et al. Involvement of mitochondrial disorders in septic cardiomyopathy. Oxidative medicine and cellular longevity. 2017;2017.
Liang D, Huang A, Jin Y, et al. Protective effects of exogenous NaHS against sepsis-induced myocardial mitochondrial injury by enhancing the PGC-1α/NRF2 pathway and mitochondrial biosynthesis in mice. American J Transl Res. 2018;10(5):1422.
Rahmel T, Marko B, Nowak H, et al. Mitochondrial dysfunction in sepsis is associated with diminished intramitochondrial TFAM despite its increased cellular expression. Sci Rep. 2020;10(1):1–11.
Carré JE, Orban J-C, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182(6):745–51.
Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+-and redox-dependent production of nitric oxide. J Immunol. 2003;171(10):5188–97.
Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev. 2016;68(1):20–48.
Lancel S, Hassoun SM, Favory R, et al. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;329(2):641–8.
Wang X, Qin W, Qiu X, et al. A novel role of exogenous carbon monoxide on protecting cardiac function and improving survival against sepsis via mitochondrial energetic metabolism pathway. Int J Biol Sci. 2014;10(7):777.
Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120(2):299–308.
Mattingly KA, Ivanova MM, Riggs KA, et al. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol. 2008;22(3):609–22.
Li Y, Feng Y-F, Liu X-T, et al. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021;38:101771.
Hondares E, Pineda-Torra I, Iglesias R, et al. PPARδ, but not PPARα, activates PGC-1α gene transcription in muscle. Biochem Biophys Res Commun. 2007;354(4):1021–7.
Yang Y, Zhu Y, Xiao J, et al. Maresin conjugates in tissue regeneration 1 prevents lipopolysaccharide-induced cardiac dysfunction through improvement of mitochondrial biogenesis and function. Biochem Pharmacol. 2020;177:114005.
Sánchez-Villamil JP, D’Annunzio V, Finocchietto P, et al. Cardiac-specific overexpression of thioredoxin 1 attenuates mitochondrial and myocardial dysfunction in septic mice. Int J Biochem Cell Biol. 2016;81:323–34.
Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127(6):1109–22.
Xin T, Lu C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY). 2020;12(16):16224.
Russell LK, Mansfield CM, Lehman JJ, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator-1α promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94(4):525–33.
Stanzani G, Duchen MR, Singer M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochimica et Biophysica Acta (BBA)- Molecular Basis of Disease. 2019;1865(4):759–73.
Ni H-M, Williams JA, Ding W-X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13.
Marín-García J, Akhmedov AT. Mitochondrial dynamics and cell death in heart failure. Heart Fail Rev. 2016;21(2):123–36.
Jin J-Y, Wei X-X, Zhi X-L, Wang X-H, Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacologica Sinica. 2021;42(5):655–64.
Ishihara T, Ban-Ishihara R, Maeda M, et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol. 2015;35(1):211–23.
Chen H, Ren S, Clish C, et al. Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J Cell Biol. 2015;211(4):795–805.
Disatnik MH, Ferreira JC, Campos JC, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2(5):e000461.
Zhou H, Zhang Y, Hu S, et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC 1-HK 2-mPTP-mitophagy axis. J Pineal Res. 2017;63(1):e12413.
Hernandez-Resendiz S, Prunier F, Girao H, et al. Targeting mitochondrial fusion and fission proteins for cardioprotection. J Cell Mol Med. 2020;24(12):6571–85.
Zang QS, Sadek H, Maass DL, et al. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. American J Physiol-Heart Circ Physiol. 2012;302(9):H1847–59.
Wu Y, Yao Y-M, Lu Z-Q. Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J Mol Med. 2019;97(4):451–62.
Gonzalez AS, Elguero ME, Finocchietto P, et al. Abnormal mitochondrial fusion–fission balance contributes to the progression of experimental sepsis. Free Radical Res. 2014;48(7):769–83.
Gao D, Zhang L, Dhillon R, et al. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS ONE. 2013;8(4):e60967.
Wu D, Dasgupta A, Chen KH, et al. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: Therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 2020;34(1):1447–64.
Tan Y, Ouyang H, Xiao X, Zhong J, Dong M. Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperones. 2019;24(3):595–608.
Yu W, Mei X, Zhang Q, et al. Yap overexpression attenuates septic cardiomyopathy by inhibiting DRP1-related mitochondrial fission and activating the ERK signaling pathway. J Recept Signal Transduct Res. 2019;39(2):175–86.
Pride CK, Mo L, Quesnelle K, et al. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc Res. 2014;101(1):57–68.
Bian X, Xu J, Zhao H, et al. Zinc-induced SUMOylation of dynamin-related protein 1 protects the heart against ischemia-reperfusion injury. Oxidative medicine and cellular longevity. 2019;2019.
Ikeda Y, Shirakabe A, Maejima Y, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116(2):264–78.
Wang J, Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia–reperfusion injury. Acta Pharmaceutica Sinica B. 2020;10(10):1866–79.
Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–32.
Ferreira JCB, Campos JC, Qvit N, et al. A selective inhibitor of mitofusin 1-βIIPKC association improves heart failure outcome in rats. Nat Commun. 2019;10(1):329.
Chen Y, Dorn GW 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340(6131):471–5.
Burke N, Hall AR, Hausenloy DJ. OPA1 in Cardiovascular Health and Disease. Curr Drug Targets. 2015;16(8):912–20.
Huang J, Li R, Wang C. The Role of Mitochondrial Quality Control in Cardiac Ischemia/Reperfusion Injury. Oxidative Med Cellular Longevity. 2021;2021.
Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280(26):25060–70.
Papanicolaou KN, Khairallah RJ, Ngoh GA, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31(6):1309–28.
Dorn GW, Clark CF, Eschenbacher WH, et al. MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res. 2011;108(1):12–7.
Chen L, Liu T, Tran A, et al. OPA 1 Mutation and Late-Onset Cardiomyopathy: Mitochondrial Dysfunction and mtDNA Instability. J Am Heart Assoc. 2012;1(5):e003012.
Merkwirth C, Dargazanli S, Tatsuta T, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22(4):476–88.
Zhang Y, Wang Y, Xu J, et al. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res. 2019;66(2):e12542.
Maneechote C, Palee S, Kerdphoo S, et al. Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci. 2019;133(3):497–513.
Shen T, Zheng M, Cao C, et al. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem. 2007;282(32):23354–61.
Ong S-B, Subrayan S, Lim SY, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–22.
Boyman L, Karbowski M, Lederer WJ. Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol Med. 2020;26(1):21–39.
Brealey D, Singer M. Mitochondrial dysfunction in sepsis. Current Infectious Disease Rep. 2003;5(5):365–71.
Leite HP, de Lima LFP. Metabolic resuscitation in sepsis: a necessary step beyond the hemodynamic? J Thorac Dis. 2016;8(7):E552.
Giacalone M, Martinelli R, Abramo A, et al. Rapid reversal of severe lactic acidosis after thiamine administration in critically ill adults: a report of 3 cases. Nutr Clin Pract. 2015;30(1):104–10.
Escames G, López LC, Ortiz F, et al. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007;274(8):2135–47.
Galley HF, Lowes DA, Allen L, et al. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. 2014;56(4):427–38.
McCall CE, Zabalawi M, Liu T, et al. Pyruvate dehydrogenase complex stimulation promotes immunometabolic homeostasis and sepsis survival. JCI insight. 2018;3:15.
Stacpoole PW, Wright EC, Baumgartner TG, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. N Engl J Med. 1992;327(22):1564–9.
Eyenga P, Roussel D, Rey B, et al. Mechanical ventilation preserves diaphragm mitochondrial function in a rat sepsis model. Intensive Care Med Exp. 2021;9(1):19.
Geng N, Ren L, Xu L, Zou D, Pang W. Clinical outcomes of nicorandil administration in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2021;21(1):488.
Bank HV, Hurtado-Thiele M, Oshimura N, Simcox J. Mitochondrial Lipid Signaling and Adaptive Thermogenesis. Metabolites. 2021;11(2):124.
Xie C, Zhang Y, Tran TD, et al. Irisin controls growth, intracellular Ca2+ signals, and mitochondrial thermogenesis in cardiomyoblasts. PLoS ONE. 2015;10(8):e0136816.
Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE. 2010;5(7):e11707.
Demine S, Renard P, Arnould T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells. 2019;8(8):795.
Tian XY, Ma S, Tse G, Wong WT, Huang Y. Uncoupling protein 2 in cardiovascular health and disease. Front Physiol. 2018;9:1060.
Chen Huang J-D, Lyu S-L, Liu J-J, Zeng C, Q-Y. Correlation between uncoupling protein 2 expression and myocardial mitochondrial injury in rats with sepsis induced by lipopolysaccharide Zhongguo dang dai er ke za zhi. Chinese J Contemporary Pediatr. 2016;18(2):159–64.
Huang J, Peng W, Zheng Y, et al. Upregulation of UCP2 expression protects against LPS-induced oxidative stress and apoptosis in cardiomyocytes. Oxidative medicine and cellular longevity. 2019;2019.
Zheng G, Lyu J, Liu S, et al. Silencing of uncoupling protein 2 by small interfering RNA aggravates mitochondrial dysfunction in cardiomyocytes under septic conditions. Int J Mol Med. 2015;35(6):1525–36.
Chen Y, Chen G, Zhang J, et al. Uncoupling protein 2 facilitates insulin-elicited protection against lipopolysaccharide-induced myocardial dysfunction. Mater Express. 2020;10(3):337–49.
Kovacic P, Pozos RS, Somanathan R, Shangari N, O’Brien PJ. Mechanism of mitochondrial uncouplers, inhibitors, and toxins: focus on electron transfer, free radicals, and structure-activity relationships. Curr Med Chem. 2005;12(22):2601–23.
Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab. 2012;23(9):477–87.
Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–90.
Ganten D, Ruckpaul K. Encyclopedic reference of genomics and proteomics in molecular medicine. Springer; 2006.
Kumar S, Gupta E, Srivastava VK, et al. Nitrosative stress and cytokines are linked with the severity of sepsis and organ dysfunction. Br J Biomed Sci. 2019;76(1):29–34.
MatÉs JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32(8):595–603.
Supinski GS, Callahan LA. Polyethylene glycol–superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am J Respir Crit Care Med. 2006;173(11):1240–7.
Liu Y, Yang W, Sun X, et al. SS31 ameliorates sepsis-induced heart injury by inhibiting oxidative stress and inflammation. Inflammation. 2019;42(6):2170–80.
Wang Z, Bu L, Yang P, Feng S, Xu F. Alleviation of sepsis-induced cardiac dysfunction by overexpression of Sestrin2 is associated with inhibition of p-S6K and activation of the p-AMPK pathway. Mol Med Rep. 2019;20(3):2511–8.
Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304(5670):596–600.
Abitagaoglu S, Akinci S, Saricaoglu F, et al. Effect of Coenzyme Q10 on Organ Damage in Sepsis. Bratisl Lek Listy. 2015;116(7):433–9.
Soltani R, Alikiaie B, Shafiee F, Amiri H, Mousavi S. Coenzyme Q10 improves the survival and reduces inflammatory markers in septic patients. Bratisl Lek Listy. 2020;121(2):154–8.
Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45(11):1559–65.
Kokkinaki D, Hoffman M, Kalliora C, et al. Chemically synthesized Secoisolariciresinol diglucoside (LGM2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J Mol Cell Cardiol. 2019;127:232–45.
Mattox TA, Psaltis C, Weihbrecht K, et al. Prohibitin-1 Is a Dynamically Regulated Blood Protein With Cardioprotective Effects in Sepsis. J Am Heart Assoc. 2021;10(14):e019877.
Lee M-T, Jung S-Y, Baek MS, Shin J, Kim W-Y. Early Vitamin C, Hydrocortisone, and Thiamine Treatment for Septic Cardiomyopathy: A Propensity Score Analysis. J Personalized Med. 2021;11(7):610.
Hobai IA, Edgecomb J, LaBarge K, Colucci WS. Dysregulation of intracellular calcium transporters in animal models of sepsis induced cardiomyopathy. Shock (Augusta, Ga). 2015;43(1):3.
Hassoun SM, Marechal X, Montaigne D, et al. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med. 2008;36(9):2590–6.
Joseph LC, Kokkinaki D, Valenti M-C, et al. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI insight. 2017;2:17.
Zhou Q, Xie M, Zhu J, et al. PINK1 Contained in huMSCs-Exosomes Prevents Cardiomyocyte Mitochondrial Calcium Overload in Sepsis by Recovering Mitochondrial Ca2+ Efflux. 2020.
Wang L, Wei Y. The Improvements of Cardiac Calcium Handing and Cardiomyopathy in Septic Rats via Nos Signaling by Neuregulin-1. Circulation. 2018;138(Suppl_1):A11603-A.
Wiewel MA, Van Vught LA, Scicluna BP, et al. Prior use of calcium channel blockers is associated with decreased mortality in critically ill patients with sepsis: a prospective observational study. Crit Care Med. 2017;45(3):454–63.
Shang X, Lin K, Yu R, et al. Resveratrol Protects the Myocardium in Sepsis by Activating the Phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway and Inhibiting the Nuclear Factor-κB (NF-κB) Signaling Pathway. Med Sci Monit. 2019;25:9290–8.
Smeding L, Leong-Poi H, Hu P, et al. Salutary effect of resveratrol on sepsis-induced myocardial depression. Crit Care Med. 2012;40(6):1896–907.
Harrington JS, Choi AMK, Nakahira K. Mitochondrial DNA in Sepsis. Curr Opin Crit Care. 2017;23(4):284–90.
West AP, Khoury-Hanold W, Staron M, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520(7548):553–7.
Kung CT, Hsiao SY, Tsai TC, et al. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med. 2012;10:130.
Jung SS, Moon JS, Xu JF, et al. Carbon monoxide negatively regulates NLRP3 inflammasome activation in macrophages. Am J Physiol Lung Cell Mol Physiol. 2015;308(10):L1058–67.
Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14.
Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–5.
Yao X, Carlson D, Sun Y, et al. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS ONE. 2015;10(10):e0139416.
Bonekamp NA, Larsson NG. SnapShot Mitochondrial Nucleoid. Cell. 2018;172(1–2):388-e1.
Yin X, Xin H, Mao S, Wu G, Guo L. The role of autophagy in sepsis: protection and injury to organs. Front Physiol. 2019;10:1071.
Queliconi BB, Kowaltowski AJ, Gottlieb RA. Bicarbonate increases ischemia-reperfusion damage by inhibiting mitophagy. PLoS ONE. 2016;11(12):e0167678.
Lu W, Sun J, Yoon JS, et al. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS ONE. 2016;11(1):e0147792.
Hertz NT, Berthet A, Sos ML, et al. A neo-substrate that amplifies catalytic activity of parkinson’s-disease-related kinase PINK1. Cell. 2013;154(4):737–47.
Green DR, Van Houten B. Mitochondrial quality control. Cell. 2011;147(4):950.
Geisler S, Holmström KM, Skujat D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–31.
Ordureau A, Sarraf SA, Duda DM, et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014;56(3):360–75.
Sedlackova L, Korolchuk VI. Mitochondrial quality control as a key determinant of cell survival. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res. 2019;1866(4):575–87.
Wei Y, Chiang WC, Sumpter R, et al. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell. 2017;168(1–2):224-38.e10.
Yun J, Puri R, Yang H, et al. MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. Elife. 2014;3:e01958.
Cadete VJ, Vasam G, Menzies KJ, Burelle Y. 2019 Mitochondrial quality control in the cardiac system An integrative view. Biochimica et Biophysica Acta (BBA)-Molecular Basis Disease. 1865;4:782–96.
Liu L, Feng D, Chen G, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14(2):177–85.
Yoo S-M, Jung Y-K. A molecular approach to mitophagy and mitochondrial dynamics. Mol Cells. 2018;41(1):18.
Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16(7):939–46.
Murakawa T, Yamaguchi O, Hashimoto A, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6:7527.
Chu CT, Ji J, Dagda RK, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15(10):1197–205.
Zhang E, Zhao X, Zhang L, et al. Minocycline promotes cardiomyocyte mitochondrial autophagy and cardiomyocyte autophagy to prevent sepsis-induced cardiac dysfunction by Akt/mTOR signaling. Apoptosis. 2019;24(3):369–81.
Cao Y, Han X, Pan H, et al. Emerging protective roles of shengmai injection in septic cardiomyopathy in mice by inducing myocardial mitochondrial autophagy via caspase-3/Beclin-1 axis. Inflamm Res. 2020;69(1):41–50.
Andres AM, Hernandez G, Lee P, et al. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal. 2014;21(14):1960–73.
Wang S, Zhao Z, Feng X, et al. Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J Cell Mol Med. 2018;22(10):5132–44.
Hsieh C-H, Pai P-Y, Hsueh H-W, Yuan S-S, Hsieh Y-C. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann Surg. 2011;253(6):1190–200.
Jiang X, Cai S, Jin Y, et al. Irisin Attenuates Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in the H9C2 Cellular Model of Septic Cardiomyopathy through Augmenting Fundc1-Dependent Mitophagy. Oxidative Medicine and Cellular Longevity. 2021;2021.
Bian X, Teng T, Zhao H, et al. Zinc prevents mitochondrial superoxide generation by inducing mitophagy in the setting of hypoxia/reoxygenation in cardiac cells. Free Radical Res. 2018;52(1):80–91.
Jang S-y, Kang HT, Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biological Chemistry. 2012;287(23):19304–14.
Ji W, Wan T, Zhang F, et al. Aldehyde Dehydrogenase 2 Protects Against Lipopolysaccharide-Induced Myocardial Injury by Suppressing Mitophagy. Front Pharmacol. 2021;12:1025.
Roshanravan B, Liu SZ, Ali AS, et al. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PLoS ONE. 2021;16(7):e0253849.
Hortmann M, Robinson S, Mohr M, et al. The mitochondria-targeting peptide elamipretide diminishes circulating HtrA2 in ST-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2019;8(8):695–702.
Daubert MA, Yow E, Dunn G, et al. Novel mitochondria-targeting peptide in heart failure treatment a randomized, placebo-controlled trial of elamipretide. Circ Heart Failure. 2017;10(12):e004389.
Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–87.
Patel BM, Chittock DR, Russell JA, Walley KR. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. 2002;96(3):576–82.
Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683–91.
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017;151(6):1229–38.
Antcliffe DB, Santhakumaran S, Orme RML, et al. Levosimendan in septic shock in patients with biochemical evidence of cardiac dysfunction: a subgroup analysis of the LeoPARDS randomised trial. Intensive Care Med. 2019;45(10):1392–400.
Kim WY, Baek MS, Kim YS, et al. Glucose-insulin-potassium correlates with hemodynamic improvement in patients with septic myocardial dysfunction. J Thorac Dis. 2016;8(12):3648–57.
Hernandez G, Bruhn A, Luengo C, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013;39(8):1435–43.
Kopterides P, Falagas M. Statins for sepsis: a critical and updated review. Clin Microbiol Infect. 2009;15(4):325–34.
Funding
This work was supported by the National Natural Sciences Foundation of China (No. 81770490), the Planned Science and Technology Project of Hunan Province, China (No.2020JJ4535), and the Key Laboratory for Arteriosclerology of Hunan Province (Basic Medicine Sciences in University of South China).
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript’s conception and design. Material preparation, literature collection, and analysis were performed by Salami Oluwabukunmi Modupe, Habimana Olive, and Jinfu Peng. Guang-hui Yi gave the outline of the manuscript and continually gave intellectual suggestions for the article.
Corresponding author
Ethics declarations
Ethics Approval
This is a review article, not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salami, O.M., Habimana, O., Peng, Jf. et al. Therapeutic Strategies Targeting Mitochondrial Dysfunction in Sepsis-induced Cardiomyopathy. Cardiovasc Drugs Ther 38, 163–180 (2024). https://doi.org/10.1007/s10557-022-07354-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07354-8