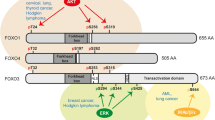
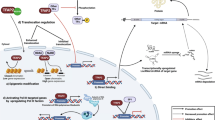
Abstract
Epigenetic regulation of gene expression is a fundamental determinant of molecular and cellular function, and epigenetic reprogramming in the context of cancer has emerged as one of the key enabling characteristics associated with acquisition of the core hallmarks of this disease. As such, there has been renewed interest in studying the role of transcription factors as epigenetic regulators of gene expression in cancer. In this review, we discuss the current state of knowledge surrounding the oncogenic functions of FOXC2, a transcription factor that frequently becomes dysregulated in a variety of cancer types. In addition to highlighting the clinical impact of aberrant FOXC2 activity in cancer, we discuss mechanisms by which this transcription factor becomes dysregulated in both tumor and tumor-associated cells, placing particular emphasis on the ways in which FOXC2 promotes key hallmarks of cancer progression. Finally, we bring attention to important issues related to the oncogenic dysregulation of FOXC2 that must be addressed going forward in order to improve our understanding of FOXC2-mediated cancer progression and to guide prognostic and therapeutic applications of this knowledge in clinical settings.


Similar content being viewed by others
References
Miura, N., Wanaka, A., Tohyama, M., & Tanaka, K. (1993). MFH-1, a new member of the fork head domain family, is expressed in developing mesenchyme. FEBS Letters, 326(1–3), 171–176. https://doi.org/10.1016/0014-5793(93)81785-X
Xue, Y., Cao, R., Nilsson, D., Chen, S., Westergren, R., Hedlund, E. M., et al. (2008). FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proceedings of the National Academy of Sciences of the United States of America, 105(29), 10167–10172. https://doi.org/10.1073/pnas.0802486105
Hader, C., Marlier, A., & Cantley, L. (2010). Mesenchymal-epithelial transition in epithelial response to injury: The role of Foxc2. Oncogene, 29(7), 1031–1040. https://doi.org/10.1038/onc.2009.397
Motojima, M., Kume, T., & Matsusaka, T. (2017). Foxc1 and Foxc2 are necessary to maintain glomerular podocytes. Experimental Cell Research, 352(2), 265–272. https://doi.org/10.1016/J.YEXCR.2017.02.016
Cederberg, A., Gronning, L. M., Ahrén, B., Taskén, K., Carlsson, P., & Enerbäck, S. (2001). FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell, 106(5), 563–573. https://doi.org/10.1016/S0092-8674(01)00474-3
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013
Hanahan, D. (2022). Hallmarks of Cancer: New dimensions. Cancer Discovery, 12(1), 31–46. https://doi.org/10.1158/2159-8290.CD-21-1059
Grønning, L. M., Cederberg, A., Miura, N., Enerbäck, S., & Taskén, K. (2002). Insulin and TNF alpha induce expression of the forkhead transcription factor gene Foxc2 in 3T3-L1 adipocytes via PI3K and ERK 1/2-dependent pathways. Molecular Endocrinology, 16(4), 873–883. https://doi.org/10.1210/MEND.16.4.0803
Paranjape, A. N., Soundararajan, R., Werden, S. J., Joseph, R., Taube, J. H., Liu, H., et al. (2016). Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene, 35(46), 5963–5976. https://doi.org/10.1038/onc.2015.498
Christofides, A., Papagregoriou, G., Dweep, H., Makrides, N., Gretz, N., Felekkis, K., & Deltas, C. (2020). Evidence for miR-548c-5p regulation of FOXC2 transcription through a distal genomic target site in human podocytes. Cellular and Molecular Life Sciences, 77(12), 2441–2459. https://doi.org/10.1007/S00018-019-03294-Z
Ge, J., Li, J., Na, S., Wang, P., Zhao, G., & Zhang, X. (2019). miR-548c-5p inhibits colorectal cancer cell proliferation by targeting PGK1. Journal of Cellular Physiology, 234(10), 18872–18878. https://doi.org/10.1002/JCP.28525
He, S. Z., & Wang, Q. (2020). MicroRNA-548c-5p inhibits the proliferation of breast cancer cells through regulating Wnt/β-catenin signaling pathway. European Review for Medical and Pharmacological Sciences, 24(7), 3795–3804. https://doi.org/10.26355/EURREV_202004_20845
Chen, C., Chen, Q., Wu, J., & Zou, H. (2021). H3K27ac-induced FOXC2-AS1 accelerates tongue squamous cell carcinoma by upregulating E2F3. Journal of OralPpathology & Medicine, 50(10), 1018–1030. https://doi.org/10.1111/JOP.13232
Lim, Y. H., Ryu, J., Kook, H., & Kim, Y. K. (2020). Identification of long noncoding RNAs involved in differentiation and survival of vascular smooth muscle cells. Molecular Therapy Nucleic Acids, 22, 209–221. https://doi.org/10.1016/J.OMTN.2020.08.032
Zhang, C.-L., Zhu, K.-P., & Ma, X.-L. (2017). Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Letters, 396, 66–75. https://doi.org/10.1016/j.canlet.2017.03.018
Pan, K., & Xie, Y. (2020). LncRNA FOXC2-AS1 enhances FOXC2 mRNA stability to promote colorectal cancer progression via activation of Ca 2+-FAK signal pathway. Cell Death & Disease, 11(6), 434. https://doi.org/10.1038/S41419-020-2633-7
Yan, J., Liu, J., Huang, Z., Huang, W., & Lv, J. (2021). FOXC2-AS1 stabilizes FOXC2 mRNA via association with NSUN2 in gastric cancer cells. Human Cell, 34(6), 1755–1764. https://doi.org/10.1007/S13577-021-00583-3
Gong, Y. Q., Ni, J. L., Fang, Q., & Li, T. (2020). MiR-1231 enhances docetaxel sensitivity to gallbladder carcinoma cells by downregulating FOXC2. European Review for Medical and Pharmacological Sciences, 24(23), 12116–12123. https://doi.org/10.26355/EURREV_202012_24000
Yan, M., Gao, H., Lv, Z., Liu, Y., Zhao, S., Gong, W., & Liu, W. (2020). Circular RNA PVT1 promotes metastasis via regulating of miR-526b/FOXC2 signals in OS cells. Journal of Cellular and Molecular Medicine, 24(10), 5593–5604. https://doi.org/10.1111/JCMM.15215
Weng, Z., Peng, J., Wu, W., Zhang, C., Zhao, J., & Gao, H. (2021). Downregulation of PART1 inhibits proliferation and differentiation of Hep3B cells by targeting hsa-miR-3529-3p/FOXC2 axis. Journal of Oncology, 2021, 7792223. https://doi.org/10.1155/2021/7792223
Liu, H., Zhang, Z., Han, Y., Fan, A., Liu, H., Zhang, X., et al. (2021). The FENDRR/FOXC2 axis contributes to multidrug resistance in gastric cancer and correlates with poor prognosis. Frontiers in Oncology, 11, 634579. https://doi.org/10.3389/FONC.2021.634579
Bi, Y., Guo, S., Xu, X., Kong, P., Cui, H., Yan, T., et al. (2020). Decreased ZNF750 promotes angiogenesis in a paracrine manner via activating DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma. Cell Death & Disease, 11(4), 296. https://doi.org/10.1038/S41419-020-2492-2
Shen, X., Zhao, K., Xu, L., Cheng, G., Zhu, J., Gan, L., et al. (2021). YTHDF2 inhibits gastric cancer cell growth by regulating FOXC2 signaling pathway. Frontiers in Genetics, 11, 592042. https://doi.org/10.3389/FGENE.2020.592042
Missaglia, S., Tavian, D., Michelini, S., Maltese, P. E., Bonanomi, A., & Bertelli, M. (2021). Imbalance between expression of FOXC2 and its lncRNA in lymphedema-distichiasis caused by frameshift mutations. Genes, 12(5), 650. https://doi.org/10.3390/GENES12050650
Golden, D., & Cantley, L. G. (2015). Casein kinase 2 prevents mesenchymal transformation by maintaining Foxc2 in the cytoplasm. Oncogene, 34(36), 4702–4712. https://doi.org/10.1038/onc.2014.395
Ivanov, K. I., Agalarov, Y., Valmu, L., Samuilova, O., Liebl, J., Houhou, N., et al. (2013). Phosphorylation regulates FOXC2-mediated transcription in lymphatic endothelial cells. Molecular and Cellular Biology, 33(19), 3749–3761. https://doi.org/10.1128/MCB.01387-12
Werden, S. J., Sphyris, N., Sarkar, T. R., Paranjape, A. N., LaBaff, A. M., Taube, J. H., et al. (2016). Phosphorylation of serine 367 of FOXC2 by p38 regulates ZEB1 and breast cancer metastasis, without impacting primary tumor growth. Oncogene, 35(46), 5977–5988. https://doi.org/10.1038/onc.2016.203
Danciu, T. E., Chupreta, S., Cruz, O., Fox, J. E., Whitman, M., & Iñiguez-Lluhí, J. A. (2012). Small ubiquitin-like modifier (SUMO) modification mediates function of the inhibitory domains of developmental regulators FOXC1 and FOXC2. The Journal of Biological Chemistry, 287(22), 18318–18329. https://doi.org/10.1074/JBC.M112.339424
Ren, Y. H., Liu, K. J., Wang, M., Yu, Y. N., Yang, K., Chen, Q., et al. (2014). De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget, 5(16), 7093–7104. https://doi.org/10.18632/ONCOTARGET.2197
Wu, D., Ke, Y., Xiao, R., Liu, J., Li, Q., & Wang, Y. (2021). Long non-coding RNA GClnc1 knockdown suppresses progression of epithelial ovarian cancer by recruiting FOXC2 to disrupt the NOTCH1/NF-κB/Snail pathway. Experimental Cell Research, 399(1). https://doi.org/10.1016/J.YEXCR.2020.112422
Zhou, P., Li, Y., Di, R., Yang, Y., Meng, S., Song, F., & Ma, L. (2019). H19 and Foxc2 synergistically promotes osteogenic differentiation of BMSCs via Wnt-β-catenin pathway. Journal of Cellular Physiology, 234(8), 13799–13806. https://doi.org/10.1002/JCP.28060
Kume, T., & Shackour, T. (2018). Meta-analysis of the likelihood of FOXC2 expression in earlyand late-stage tumors. Oncotarget, 9(70), 33396–33402. https://doi.org/10.18632/oncotarget.26087
Li, Y., Yang, W., Yang, Q., & Zhou, S. (2012). Nuclear localization of GLI1 and elevated expression of FOXC2 in breast cancer is associated with the basal-like phenotype. Histology and Histopathology, 27(4), 475–84. https://doi.org/10.14670/HH-27.475
Li, Q., Wu, J., Wei, P., Xu, Y., Zhuo, C., Wang, Y., et al. (2015). Overexpression of forkhead Box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial-mesenchymal transition. American Journal of Cancer Research, 5(6), 2022–34. http://www.ncbi.nlm.nih.gov/pubmed/26269761
Jiang, W., Fan, H., Qian, C., Ding, J., Wang, Q., & Pang, X. (2016). Prognostic value of high FoxC2 expression in resectable non-small cell lung cancer, alone or in combination with E-cadherin expression. BMC Cancer, 16(1), 16. https://doi.org/10.1186/s12885-016-2056-0
Shimoda, Y., Ubukata, Y., Handa, T., Yokobori, T., Watanabe, T., Gantumur, D., et al. (2018). High expression of forkhead box protein C2 is associated with aggressive phenotypes and poor prognosis in clinical hepatocellular carcinoma. BMC Cancer, 18(1), 597. https://doi.org/10.1186/s12885-018-4503-6
Børretzen, A., Gravdal, K., Haukaas, S. A., Beisland, C., Akslen, L. A., & Halvorsen, O. J. (2019). FOXC2 expression and epithelial–mesenchymal phenotypes are associated with castration resistance, metastasis and survival in prostate cancer. The Journal of Pathology: Clinical Research, 5(4), 272–286. https://doi.org/10.1002/cjp2.142
Zhu, J.-L., Song, Y.-X., Wang, Z.-N., Gao, P., Wang, M.-X., Dong, Y.-L., et al. (2013). The clinical significance of mesenchyme forkhead 1 (FoxC2) in gastric carcinoma. Histopathology, 62(7), 1038–1048. https://doi.org/10.1111/his.12132
Xu, B., Tian, Y., & Liu, L. (2020). Meta-analysis of the prognostic significance of FOXC2 in various tumors. The Journal of International Medical Research, 48(3), 300060519891648. https://doi.org/10.1177/0300060519891648
Hargadon, K. M., Győrffy, B., & Strong, E. W. (2021). The prognostic significance of FOXC2 gene expression in cancer: A comprehensive analysis of RNA-seq data from the cancer genome atlas. Cancer genetics, 254–255, 58–64. https://doi.org/10.1016/J.CANCERGEN.2021.02.005
Nishida, N., Mimori, K., Yokobori, T., Sudo, T., Tanaka, F., Shibata, K., et al. (2011). FOXC2 is a novel prognostic factor in human esophageal squamous cell carcinoma. Annals of Surgical Oncology, 18(2), 535–542. https://doi.org/10.1245/s10434-010-1274-y
Wang, Y.-W., Yin, C.-L., Zhang, H.-Y., Hao, J., Yang, Y.-Y., Liao, H., & Jiao, B.-H. (2014). High expression of forkhead box protein C2 is related to poor prognosis in human gliomas. Asian Pacific Journal of Cancer Prevention, 15(24), 10621–10625. https://doi.org/10.7314/apjcp.2014.15.24.10621
Hargadon, K. M., Györffy, B., Strong, E. W., Tarnai, B. D., Thompson, J. C., Bushhouse, D. Z., et al. (2019). The FOXC2 transcription factor promotes melanoma outgrowth and regulates expression of genes associated with drug resistance and interferon responsiveness. Cancer Genomics & Proteomics, 16(6), 491–503. https://doi.org/10.21873/cgp.20152
Yang, H., Chen, T., Xu, S., Zhang, S., & Zhang, M. (2019). Long noncoding RNA FOXC2-AS1 predicts poor survival in breast cancer patients and promotes cell proliferation. Oncology Research, 27(2), 219–226. https://doi.org/10.3727/096504018X15213126075068
Xu, D. F., Tao, X. H., Yu, Y., Teng, Y., Huang, Y. M., Ma, J. W., & Fan, Y. B. (2020). LncRNA FOXC2-AS1 stimulates proliferation of melanoma via silencing p15 by recruiting EZH2. European Review for Medical and Pharmacological Sciences, 24(17), 8940–8946. https://doi.org/10.26355/EURREV_202009_22835
Cui, Y.-M., Jiang, D., Zhang, S.-H., Wu, P., Ye, Y.-P., Chen, C.-M., et al. (2014). FOXC2 promotes colorectal cancer proliferation through inhibition of FOXO3a and activation of MAPK and AKT signaling pathways. Cancer Letters, 353(1), 87–94. https://doi.org/10.1016/j.canlet.2014.07.008
RS, N., & P, H. (2014). FoxO3a and disease progression. World Journal of Biological Chemistry, 5(3), 346. https://doi.org/10.4331/WJBC.V5.I3.346
Park, S. J., Gadi, J., Cho, K. W., Kim, K. J., Kim, S. H., Jung, H. S., & Lim, S. K. (2011). The forkhead transcription factor Foxc2 promotes osteoblastogenesis via up-regulation of integrin β1 expression. Bone, 49(3), 428–438. https://doi.org/10.1016/J.BONE.2011.05.012
Pan, J., Yang, Q., Shao, J., Zhang, L., Ma, J., Wang, Y., et al. (2016). Cyclooxygenase-2 induced β1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway. Scientific Reports, 6, 33823. https://doi.org/10.1038/srep33823
Shibue, T., & Weinberg, R. A. (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences of the United States of America, 106(25), 10290–10295. https://doi.org/10.1073/PNAS.0904227106
Cui, L., Dang, S., Qu, J., Mao, Z., Wang, X., Zhang, J., & Chen, J. (2016). FOXC2 is up-regulated in pancreatic ductal adenocarcinoma and promotes the growth and migration of cancer cells. Tumour Biology, 37(7), 8579–8585. https://doi.org/10.1007/S13277-015-4607-4
Gozo, M. C., Aspuria, P. J., Cheon, D. J., Walts, A. E., Berel, D., Miura, N., et al. (2013). Foxc2 induces Wnt4 and Bmp4 expression during muscle regeneration and osteogenesis. Cell Death and Differentiation, 20(8), 1031. https://doi.org/10.1038/CDD.2013.34
Li, W., Fu, X., Liu, R., Wu, C., Bai, J., Xu, Y., et al. (2013). FOXC2 often overexpressed in glioblastoma enhances proliferation and invasion in glioblastoma cells. Oncology Research, 21(2), 111–120. https://doi.org/10.3727/096504013X13814233062171
Zheng, C.-H., Quan, Y., Li, Y.-Y., Deng, W.-G., Shao, W.-J., & Fu, Y. (2014). Expression of transcription factor FOXC2 in cervical cancer and effects of silencing on cervical cancer cell proliferation. Asian Pacific Journal of Cancer Prevention, 15(4), 1589–1595. https://doi.org/10.7314/apjcp.2014.15.4.1589
Gozo, M. C., Jia, D., Aspuria, P.-J., Cheon, D.-J., Miura, N., Walts, A. E., et al. (2016). FOXC2 augments tumor propagation and metastasis in osteosarcoma. Oncotarget, 7(42), 68792–68802. https://doi.org/10.18632/oncotarget.11990
Li, C., Ding, H., Tian, J., Wu, L., Wang, Y., Xing, Y., & Chen, M. (2016). Forkhead box protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cellular Physiology and Biochemistry, 39(1), 242–252. https://doi.org/10.1159/000445620
Yang, C., Cui, X., Dai, X., & Liao, W. (2016). Downregulation of Foxc2 enhances apoptosis induced by 5-fluorouracil through activation of MAPK and AKT pathways in colorectal cancer. Oncology Letters, 11(2), 1549–1554. https://doi.org/10.3892/ol.2016.4097
Gan, L., Liu, Z., Jin, W., Zhou, Z., & Sun, C. (2015). Foxc2 enhances proliferation and inhibits apoptosis through activating Akt/mTORC1 signaling pathway in mouse preadipocytes. Journal of Lipid Research, 56(8), 1471–1480. https://doi.org/10.1194/jlr.M057679
Lambert, A. W., & Weinberg, R. A. (2021). Linking EMT programmes to normal and neoplastic epithelial stem cells. Nature Reviews Cancer, 21(5), 325–338. https://doi.org/10.1038/S41568-021-00332-6
Mani, S. A., Yang, J., Brooks, M., Schwaninger, G., Zhou, A., Miura, N., A., et al. (2007). Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proceedings of the National Academy of Sciences of the United States of America, 104(24), 10069–10074. https://doi.org/10.1073/pnas.0703900104
Li, C., Ding, H., Tian, J., Wu, L., Wang, Y., Xing, Y., & Chen, M. (2016). Forkhead box protein C2 promotes epithelial-mesenchymal transition, migration and invasion in cisplatin-resistant human ovarian cancer cell line (SKOV3/CDDP). Cellular Physiology and Biochemistry, 39(3), 1098–1110. https://doi.org/10.1159/000447818
Chen, J., Rong, X., Liu, X., Zheng, D., Rong, X., Chen, F., et al. (2020). FOXC2 is a prognostic biomarker and contributes to the growth and invasion of human hepatocellular carcinoma. Cancer Cell International, 20(1). https://doi.org/10.1186/s12935-020-01265-0
Mortazavi, F., An, J., Dubinett, S., & Rettig, M. (2010). p120-catenin is transcriptionally downregulated by FOXC2 in non-small cell lung cancer cells. Molecular Cancer Research, 8(5), 762–774. https://doi.org/10.1158/1541-7786.MCR-10-0004
Pham, T. N. D., Perez White, B. E., Zhao, H., Mortazavi, F., & Tonetti, D. A. (2017). Protein kinase C α enhances migration of breast cancer cells through FOXC2-mediated repression of p120-catenin. BMC Cancer, 17(1), 832. https://doi.org/10.1186/s12885-017-3827-y
Aster, J. C., Pear, W. S., & Blacklow, S. C. (2017). The varied roles of Notch in cancer. Annual Review of Pathology, 12, 245–275. https://doi.org/10.1146/ANNUREV-PATHOL-052016-100127
Kwon, O. J., Zhang, L., Wang, J., Su, Q., Feng, Q., Zhang, X. H. F., et al. (2016). Notch promotes tumor metastasis in a prostate-specific Pten-null mouse model. The Journal of Clinical Investigation, 126(7), 2626–2641. https://doi.org/10.1172/JCI84637
Hayashi, H., Sano, H., Seo, S., & Kume, T. (2008). The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression. The Journal of Biological Chemistry, 283(35), 23791–23800. https://doi.org/10.1074/jbc.M800190200
Hamidi, H., & Ivaska, J. (2018). Every step of the way: Integrins in cancer progression and metastasis. Nature Reviews Cancer, 18(9), 533–548. https://doi.org/10.1038/s41568-018-0038-z
Su, C. Y., Li, J. Q., Zhang, L. L., Wang, H., Wang, F. H., Tao, Y. W., et al. (2020). The biological functions and clinical applications of integrins in cancers. Frontiers in Pharmacology, 11, 579068. https://doi.org/10.3389/FPHAR.2020.579068
Watanabe, A., Suzuki, H., Yokobori, T., Altan, B., Kubo, N., Araki, K., et al. (2013). Forkhead box protein C2 contributes to invasion and metastasis of extrahepatic cholangiocarcinoma, resulting in a poor prognosis. Cancer Science, 104(11), 1427–1432. https://doi.org/10.1111/CAS.12249
Zhang, Y., & Zhang, Y. (2018). Forkhead box C2 promotes the invasion ability of human trophoblast cells through Hedgehog (Hh) signaling pathway. Cell Biology International, 42(7), 859–866. https://doi.org/10.1002/CBIN.10953
Cui, Y.-M., Jiao, H.-L., Ye, Y.-P., Chen, C.-M., Wang, J.-X., Tang, N., et al. (2015). FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene, 34(33), 4379–4390. https://doi.org/10.1038/onc.2014.368
Sarkar, T. R., Battula, V. L., Werden, S. J., Vijay, G. V., Ramirez-Peña, E. Q., Taube, J. H., et al. (2015). GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene, 34(23), 2958–2967. https://doi.org/10.1038/ONC.2014.245
Hollier, B. G., Tinnirello, A. A., Werden, S. J., Evans, K. W., Taube, J. H., Sarkar, T. R., et al. (2013). FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Research, 73(6), 1981–1992. https://doi.org/10.1158/0008-5472.CAN-12-2962
Davis, K. E., Moldes, M., & Farmer, S. R. (2004). The forkhead transcription factor FoxC2 inhibits white adipocyte differentiation. Journal of Biological Chemistry, 279(41), 42453–42461. https://doi.org/10.1074/JBC.M402197200
Kume, T. (2009). The cooperative roles of Foxc1 and Foxc2 in cardiovascular development. Advances in Experimental Medicine and Biology, 665, 63–77. https://doi.org/10.1007/978-1-4419-1599-3_5
Kume, T. (2008). Foxc2 transcription factor: A newly described regulator of angiogenesis. Trends in Cardiovascular Medicine, 18(6), 224–228. https://doi.org/10.1016/J.TCM.2008.11.003
Lin, Y., Mckinnon, K. E., Ha, S. W., & Beck, G. R. (2015). Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Molecular Carcinogenesis, 54(9), 926–934. https://doi.org/10.1002/MC.22153
Sano, H., Leboeuf, J. P., Novitskiy, S. V., Seo, S., Zaja-Milatovic, S., Dikov, M. M., & Kume, T. (2010). The Foxc2 transcription factor regulates tumor angiogenesis. Biochemical and Biophysical Research Communications, 392(2), 201–206. https://doi.org/10.1016/j.bbrc.2010.01.015
Fukumura, D., Xavier, R., Sugiura, T., Chen, Y., Park, E. C., Lu, N., et al. (1998). Tumor induction of VEGF promoter activity in stromal cells. Cell, 94(6), 715–725. https://doi.org/10.1016/S0092-8674(00)81731-6
Kume, T. (2012). The role of FoxC2 transcription factor in tumor angiogenesis. Journal of Oncology, 2012, 204593. https://doi.org/10.1155/2012/204593
Yao, H., & He, S. (2021). Multi-faceted role of cancer-associated adipocytes in the tumor microenvironment (Review). Molecular Medicine Reports, 24(6), 866. https://doi.org/10.3892/MMR.2021.12506
Hayashi, H., & Kume, T. (2008). Forkhead transcription factors regulate expression of the chemokine receptor CXCR4 in endothelial cells and CXCL12-induced cell migration. Biochemical and Biophysical Research Communications, 367(3), 584–589. https://doi.org/10.1016/J.BBRC.2007.12.183
Hayashi, H., & Kume, T. (2008). Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS ONE, 3(6), e2401. https://doi.org/10.1371/JOURNAL.PONE.0002401
Xia, S., Menden, H. L., Korfhagen, T. R., Kume, T., & Sampath, V. (2018). Endothelial immune activation programmes cell-fate decisions and angiogenesis by inducing angiogenesis regulator DLL4 through TLR4-ERK-FOXC2 signalling. The Journal of Physiology, 596(8), 1397–1417. https://doi.org/10.1113/JP275453
Fujita, H., Kang, M., Eren, M., Gleaves, L. A., Vaughan, D. E., & Kume, T. (2006). Foxc2 is a common mediator of insulin and transforming growth factor β signaling to regulate plasminogen activator inhibitor type I gene expression. Circulation Research, 98(5), 626–634. https://doi.org/10.1161/01.RES.0000207407.51752.3c
Takayama, Y., Hattori, N., Hamada, H., Masuda, T., Omori, K., Akita, S., et al. (2016). Inhibition of PAI-1 limits tumor angiogenesis regardless of angiogenic stimuli in malignant pleural mesothelioma. Cancer Research, 76(11), 3285–3294. https://doi.org/10.1158/0008-5472.CAN-15-1796
Sphyris, N., King, C., Hoar, J., Werden, S. J., Vijay, G. V., Miura, N., et al. (2021). Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth. Oncotarget, 12(8), 823–844. https://doi.org/10.18632/ONCOTARGET.27940
Hernández de la Cruz, O. N., López-González, J. S., García-Vázquez, R., Salinas-Vera, Y. M., Muñiz-Lino, M. A., Aguilar-Cazares, D., et al. (2020). Regulation networks driving vasculogenic mimicry in solid tumors. Frontiers in Oncology, 9, 1419. https://doi.org/10.3389/FONC.2019.01419
Wei, X., Chen, Y., Jiang, X., Peng, M., Liu, Y., Mo, Y., et al. (2021). Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Molecular Cancer, 20(1), 7. https://doi.org/10.1186/S12943-020-01288-1
Fang, J., Dagenais, S. L., Erickson, R. P., Arlt, M. F., Glynn, M. W., Gorski, J. L., et al. (2000). Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. American Journal of Human Genetics, 67(6), 1382–1388. https://doi.org/10.1086/316915
Tavian, D., Missaglia, S., Maltese, P. E., Michelini, S., Fiorentino, A., Ricci, M., et al. (2016). FOXC2 disease-mutations identified in lymphedema-distichiasis patients cause both loss and gain of protein function. Oncotarget, 7(34), 54228–54239. https://doi.org/10.18632/ONCOTARGET.9797
González-Loyola, A., Bovay, E., Kim, J., Lozano, T. W., Sabine, A., Renevey, F., et al. (2021). FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure. Science Advances, 7(29), eabf4335. https://doi.org/10.1126/SCIADV.ABF4335
Liebl, J., Zhang, S., Moser, M., Agalarov, Y., Demir, C. S., Hager, B., et al. (2015). Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nature Communications, 6, 7274. https://doi.org/10.1038/NCOMMS8274
Kanady, J. D., Munger, S. J., Witte, M. H., & Simon, A. M. (2015). Combining Foxc2 and Connexin37 deletions in mice leads to severe defects in lymphatic vascular growth and remodeling. Developmental Biology, 405(1), 33–46. https://doi.org/10.1016/J.YDBIO.2015.06.004
Fatima, A., Wang, Y., Uchida, Y., Norden, P., Liu, T., Culver, A., et al. (2016). Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation. The Journal of Clinical Investigation, 126(7), 2437–2451. https://doi.org/10.1172/JCI80465
Cha, B., Geng, X., Mahamud, M. R., Fu, J., Mukherjee, A., Kim, Y., et al. (2016). Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes and Development, 30(12), 1454–1469. https://doi.org/10.1101/GAD.282400.116/-/DC1
Jiang, W., Pang, X.-G., Wang, Q., Shen, Y.-X., Chen, X.-K., & Xi, J.-J. (2012). Prognostic role of Twist, Slug, and Foxc2 expression in stage I non–small-cell lung cancer after curative resection. Clinical Lung Cancer, 13(4), 280–287. https://doi.org/10.1016/j.cllc.2011.11.005
Sasahira, T., Ueda, N., Yamamoto, K., Kurihara, M., Matsushima, S., Bhawal, U. K., et al. (2014). Prox1 and FOXC2 act as regulators of lymphangiogenesis and angiogenesis in oral squamous cell carcinoma. PLoS ONE, 9(3), e92534. https://doi.org/10.1371/journal.pone.0092534
Pereira, E. R., Kedrin, D., Seano, G., Gautier, O., Meijer, E. F. J., Jones, D., P., et al. (2018). Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science, 359(6382), 1403–1407. https://doi.org/10.1126/science.aal3622
Brown, M., Assen, F. P., Leithner, A., Abe, J., Schachner, H., Asfour, G., et al. (2018). Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science, 359(6382), 1408–1411. https://doi.org/10.1126/science.aal3662
Kim, J. K., Kim, H. J., Park, S. Y., Cederberg, A., Westergren, R., Nilsson, D., et al. (2005). Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes, 54(6), 1657–1663. https://doi.org/10.2337/DIABETES.54.6.1657
Håkansson, J., Eliasson, B., Smith, U., & Enerbäck, S. (2011). Adipocyte mitochondrial genes and the forkhead factor FOXC2 are decreased in type 2 diabetes patients and normalized in response to rosiglitazone. Diabetology and Metabolic Syndrome, 3(1), 1–9. https://doi.org/10.1186/1758-5996-3-32/FIGURES/4
Nian, X., Zhang, X., Wang, Y., Li, H., Li, J., Liu, H., & Qin, L. (2016). Correlations of FOXC2 gene expression and polymorphism with type 2 diabetes mellitus. Clinical Laboratory, 62(5), 781–791. https://doi.org/10.7754/CLIN.LAB.2015.150729
Peng, Y. H., Wang, P., He, X. Q., Hong, M. Z., & Liu, F. (2022). Micro ribonucleic acid-363 regulates the phosphatidylinositol 3-kinase/threonine protein kinase axis by targeting NOTCH1 and forkhead box C2, leading to hepatic glucose and lipids metabolism disorder in type 2 diabetes mellitus. Journal of Diabetes Investigation, 13(2), 236–248. https://doi.org/10.1111/JDI.13695
Lidell, M. E., Seifert, E. L., Westergren, R., Heglind, M., Gowing, A., Sukonina, V., et al. (2011). The adipocyte-expressed forkhead transcription factor Foxc2 regulates metabolism through altered mitochondrial function. Diabetes, 60(2), 427–435. https://doi.org/10.2337/DB10-0409
Gan, L., Liu, Z., Chen, Y., Luo, D., Feng, F., Liu, G., & Sun, C. (2016). α-MSH and Foxc2 promote fatty acid oxidation through C/EBPβ negative transcription in mice adipose tissue. Scientific Reports, 6(1), 36661. https://doi.org/10.1038/srep36661
Ma, Y., Temkin, S. M., Hawkridge, A. M., Guo, C., Wang, W., Wang, X. Y., & Fang, X. (2018). Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Letters, 435, 92–100. https://doi.org/10.1016/J.CANLET.2018.08.006
DeBerardinis, R. J., & Chandel, N. S. (2016). Fundamentals of cancer metabolism. Science. Advances, 2(5), e1600200. https://doi.org/10.1126/sciadv.1600200
Song, L., Tang, H., Liao, W., Luo, X., Li, Y., Chen, T., & Zhang, X. (2017). FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Experimental Cell Research, 357(1), 17–24. https://doi.org/10.1016/j.yexcr.2017.04.019
Ramirez-Peña, E., Arnold, J., Shivakumar, V., Joseph, R., Vidhya Vijay, G., den Hollander, P., … Mani, S. A. (2019). The epithelial to mesenchymal transition promotes glutamine independence by suppressing GLS2 expression. Cancers, 11(10), 1610. https://doi.org/10.3390/cancers11101610
Liu, M., Zhong, J., Zeng, Z., Huang, K., Ye, Z., Deng, S., et al. (2019). Hypoxia-induced feedback of HIF-1α and lncRNA-CF129 contributes to pancreatic cancer progression through stabilization of p53 protein. Theranostics, 9(16), 4795–4810. https://doi.org/10.7150/THNO.30988
Robey, I. F., Lien, A. D., Welsh, S. J., Baggett, B. K., & Gillies, R. J. (2005). Hypoxia-inducible factor-1α and the glycolytic phenotype in tumors. Neoplasia, 7(4), 324. https://doi.org/10.1593/NEO.04430
Yang, L., Li, T., & Zha, L. (2020). Foxc2 alleviates ox-LDL-induced lipid accumulation, inflammation, and apoptosis of macrophage via regulating the expression of Angptl2. Inflammation, 43(4), 1397–1410. https://doi.org/10.1007/S10753-020-01217-W
Wang, R., Wu, Y., & Jiang, S. (2021). FOXC2 alleviates myocardial ischemia-reperfusion injury in rats through regulating Nrf2/HO-1 signaling pathway. Disease Markers, 2021, 9628521. https://doi.org/10.1155/2021/9628521
Hargadon, K. M., & Williams, C. J. (2020). RNA-seq analysis of wild-type vs. FOXC2-deficient melanoma cells reveals a role for the FOXC2 transcription factor in the regulation of multiple oncogenic pathways. Frontiers in Oncology, 10, 267. https://doi.org/10.3389/fonc.2020.00267
Saxena, M., Stephens, M. A., Pathak, H., & Rangarajan, A. (2011). Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death and Disease, 2(7), e179. https://doi.org/10.1038/cddis.2011.61
Zhou, Z., Zhang, L., Xie, B., Wang, X., Yang, X., Ding, N., et al. (2015). FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Letters, 363(2), 137–145. https://doi.org/10.1016/j.canlet.2015.04.008
Cai, J., Tian, A.-X., Wang, Q.-S., Kong, P.-Z., Du, X., Li, X.-Q., & Feng, Y.-M. (2015). FOXF2 suppresses the FOXC2-mediated epithelial–mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Letters, 367(2), 129–137. https://doi.org/10.1016/j.canlet.2015.07.001
He, Y., Xie, H., Yu, P., Jiang, S., & Wei, L. (2018). FOXC2 promotes epithelial–mesenchymal transition and cisplatin resistance of non-small cell lung cancer cells. Cancer Chemotherapy and Pharmacology, 82(6), 1049–1059. https://doi.org/10.1007/s00280-018-3697-2
Hargadon, K. M. (2020). Tumor microenvironmental influences on dendritic cell and T cell function: A focus on clinically relevant immunologic and metabolic checkpoints. Clinical and Translational Medicine, 10(1), 374–411. https://doi.org/10.1002/ctm2.37
Norrmén, C., Ivanov, K. I., Cheng, J., Zangger, N., Delorenzi, M., Jaquet, M., et al. (2009). FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. The Journal of Cell Biology, 185(3), 439–457. https://doi.org/10.1083/JCB.200901104
Richards, A. L., Eckhardt, M., & Krogan, N. J. (2021). Mass spectrometry-based protein-protein interaction networks for the study of human diseases. Molecular Systems Biology, 17(1), e8792. https://doi.org/10.15252/MSB.20188792
Bushweller, J. H. (2019). Targeting transcription factors in cancer—from undruggable to reality. Nature Reviews Cancer, 19(11), 611–624. https://doi.org/10.1038/s41568-019-0196-7
Castaneda, M., Chen, L., Pradhan, L., Li, S., Zein, R., Lee, Y., et al. (2018). A forkhead box protein C2 inhibitor: Targeting epithelial-mesenchymal transition and cancer metastasis. ChemBioChem, 19(13), 1359–1364. https://doi.org/10.1002/cbic.201800022
El-Ashmawy, N. E., El-Zamarany, E. A., Khedr, N. F., Selim, H. M., & Khedr, E. G. (2021). Inhibition of PKC/MEK pathway suppresses β1-integrin and mitigates breast cancer cells proliferation. Toxicology Reports, 8, 1530–1537. https://doi.org/10.1016/J.TOXREP.2021.07.012
Gong, L., Zhang, Y., Liu, C., Zhang, M., & Han, S. (2021). Application of radiosensitizers in cancer radiotherapy. International Journal of Nanomedicine, 16, 1083–1102. https://doi.org/10.2147/IJN.S290438
Imayama, N., Yamada, S.-I., Yanamoto, S., Naruse, T., Matsushita, Y., Takahashi, H., et al. (2015). FOXC2 expression is associated with tumor proliferation and invasion potential in oral tongue squamous cell carcinoma. Pathology Oncology Research, 21(3), 783–791. https://doi.org/10.1007/s12253-014-9891-6
Galván, J. A., Astudillo, A., Vallina, A., Crespo, G., Folgueras, M. V., & González, M. V. (2014). Prognostic and diagnostic value of epithelial to mesenchymal transition markers in pulmonary neuroendocrine tumors. BMC Cancer, 14(1), 855. https://doi.org/10.1186/1471-2407-14-855
Ma, L., Yang, R., Gu, J., Jiang, H., & Li, H. (2020). The expression of AGGF1, FOXC2, and E-cadherin in esophageal carcinoma and their clinical significance. Medicine, 99(37), e22173. https://doi.org/10.1097/MD.0000000000022173
Sun, Y., Wang, X., Wen, H., Zhu, B., & Yu, L. (2021). Expression and clinical significance of the NCAPH, AGGF1, and FOXC2 proteins in serous ovarian cancer. Cancer Management and Research, 13, 7253–7262. https://doi.org/10.2147/CMAR.S329688
Sun, T.-Y., Xie, H.-J., & Kong, L.-F. (2015). Expression of FOXC2 in renal cell carcinoma and its relationship to clinical pathological features. International Journal of Clinical and Experimental Medicine, 8(8), 13388–13392. https://pubmed.ncbi.nlm.nih.gov/26550271/
Zhu, Q., Tang, M., & Wu, L. (2020). Expression of combined interference of slug and FoxC2 in endometrial carcinoma and its clinicopathological relationship. Translational Cancer Research, 9(9), 5268–5280. https://doi.org/10.21037/TCR-20-809
Seo, S., Fujita, H., Nakano, A., Kang, M., Duarte, A., & Kume, T. (2006). The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Developmental Biology, 294(2), 458–470. https://doi.org/10.1016/j.ydbio.2006.03.035
Xia, S., Yu, W., Menden, H., Younger, S. T., & Sampath, V. (2021). FOXC2 autoregulates its expression in the pulmonary endothelium after endotoxin stimulation in a histone acetylation-dependent manner. Frontiers in Cell and Developmental Biology, 9, 657662. https://doi.org/10.3389/FCELL.2021.657662
Funding
Work in the Hargadon lab is currently supported by a Mary Louise Andrews Award for Cancer Research from the Virginia Academy of Science. Portions of the figures in this review were generated using BioRender software.
Author information
Authors and Affiliations
Contributions
Kristian M. Hargadon—conceptualization, literature search, supervision, writing, and manuscript/figure preparation.
Travis B. Goodloe III—literature search, organization of data for tables.
Nathaniel D. Lloyd—literature search, organization of data for tables.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hargadon, K.M., Goodloe, T.B. & Lloyd, N.D. Oncogenic functions of the FOXC2 transcription factor: a hallmarks of cancer perspective. Cancer Metastasis Rev 41, 833–852 (2022). https://doi.org/10.1007/s10555-022-10045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10045-3