Abstract
Purpose
Neoadjuvant chemotherapy (NAC) for triple-negative (TN) and Her2-positive (HER2) breast cancers is supported by international guidelines as it can decrease extent of surgery, provide prognostic information, and allow response-driven adjuvant therapies. Our goal was to describe practice patterns for patients with TN and HER2-positive breast cancer and identify the factors associated with the receipt of NAC versus surgery as initial treatment.
Methods
A retrospective population-based cohort study of adult women diagnosed with stage I–III TN or HER2-positive breast cancer (2012–2020) in Ontario was completed using linked administrative datasets. The primary outcome was NAC as first treatment. The association between NAC and patient, tumor, and practice-related factors was examined using multivariable logistic regression models.
Results
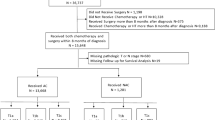
Of 14,653 patients included, 23.9% (n = 3500) underwent NAC as first treatment. Patients who underwent NAC were more likely to be younger and have larger tumors, node-positive disease, and stage 3 disease. Of patients who underwent surgery first, 8.8% were seen by a medical oncologist prior to surgery. On multivariable analysis, increasing tumor size (T2 vs T1/T0: 2.75 (2.31–3.28)) and node-positive (N1 vs N0: OR 3.54 (2.92–4.30)) disease were both associated increased odds of receiving NAC.
Conclusion
A considerable proportion of patients with TN and HER2-positive breast cancer do not receive NAC as first treatment. Of those, most were not assessed by both a surgeon and medical oncologist prior to initiating therapy. This points toward potential gaps in multidisciplinary assessment and disparities in receipt of guideline-concordant care.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to restricted access to provincial administrative datasets but may be available from the corresponding author upon reasonable request.
References
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr 30:96–102. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003469
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol 21(22):4165–4174. https://doi.org/10.1200/JCO.2003.12.005
Boughey JC, Suman VJ, Mittendorf EA et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14):1455–1461. https://doi.org/10.1001/jama.2013.278932
Boileau JF, Poirier B, Basik M et al (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33(3):258–264. https://doi.org/10.1200/JCO.2014.55.7827
Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS (2018) Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat 170(3):559–567. https://doi.org/10.1007/s10549-018-4801-3
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Fallahpour S, Navaneelan T, De P, Borgo A (2017) Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open 5(3):E734–E739. https://doi.org/10.9778/cmajo.20170030
Masuda N, Lee SJ, Ohtani S et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376(22):2147–2159. https://doi.org/10.1056/NEJMoa1612645
Von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380(7):617–628. https://doi.org/10.1056/NEJMoa1814017
Gandhi S, Brackstone M, Hong NJL et al (2022) A Canadian national guideline on the neoadjuvant treatment of invasive breast cancer, including patient assessment, systemic therapy, and local management principles. Breast Cancer Res Treat 193(1):1–20. https://doi.org/10.1007/s10549-022-06522-6
Korde LA, Somerfield MR, Carey LA et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39(13):1485–1505. https://doi.org/10.1200/JCO.20.03399
Cardoso F, Kyriakides S, Ohno S et al (2019) Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(8):1194–1220. https://doi.org/10.1093/annonc/mdz173
NICE 2018. Early and locally advanced breast cancer: diagnosis and management. https://www.nice.org.uk/guidance/ng101 /resources/early-and-locally-advanced-breast-cancer-diagnosis- and-management-pdf-66141532914901.
Brezden-Masley C, Fathers KE, Coombes ME, Pourmirza B, Xue C, Jerzak KJ (2020) A population-based comparison of treatment patterns, resource utilization, and costs by cancer stage for Ontario patients with triple-negative breast cancer. Cancer Med 9(20):7548–7557. https://doi.org/10.1002/cam4.3038
Brezden-Masley C, Fathers KE, Coombes ME, Pourmirza B, Xue C, Jerzak KJ (2021) A population-based comparison of treatment, resource utilization, and costs by cancer stage for Ontario patients with HER2-positive breast cancer. Breast Cancer Res Treat 185(3):807–815. https://doi.org/10.1007/s10549-020-05976-w
Mougalian SS, Soulos PR, Killelea BK et al (2015) Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 121(15):2544–2552. https://doi.org/10.1002/cncr.29348
Mohiuddin JJ, Deal AM, Carey LA et al (2016) Neoadjuvant systemic therapy use for younger patients with breast cancer treated in different types of cancer centers across the United States. J Am Coll Surg 223(5):717-728.e4. https://doi.org/10.1016/j.jamcollsurg.2016.08.541
Whitehead I, Irwin GW, Bannon F et al (2021) The NeST (Neoadjuvant systemic therapy in breast cancer) study: National practice questionnaire of United Kingdom multi-disciplinary decision making. BMC Cancer 21(1):90. https://doi.org/10.1186/s12885-020-07757-6
Spronk PER, Volders JH, van den Tol P, Smorenburg CH, Vrancken Peeters MJTFD (2019) Breast conserving therapy after neoadjuvant chemotherapy; data from the Dutch breast cancer audit. Eur J Surg Oncol 45(2):110–117. https://doi.org/10.1016/j.ejso.2018.09.027
Benchimol EI, Smeeth L, Guttmann A et al (2015) The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 12(10):e1001885. https://doi.org/10.1371/journal.pmed.1001885
Wodchis WP, Bushmeneva K, Nikitovic M, McKillop I (2013) Guidelines on person-level costing using administrative databases in Ontario.
Mittmann N, Seung S, Cheng S et al (2015) Generating costing algorithms for oncology drugs using administrative databases. Value in Health 18(7):A691. https://doi.org/10.1016/j.jval.2015.09.2568
Mittmann N, Cheng SY, Liu N et al (2019) The generation of two specific cancer costing algorithms using Ontario administrative databases. Curr Oncol 26(5):e682–e692. https://doi.org/10.3747/co.26.5279
Mavros MN, Coburn NG, Davis LE et al (2019) Low rates of specialized cancer consultation and cancer-directed therapy for noncurable pancreatic adenocarcinoma: a population-based analysis. CMAJ 191(21):E574–E580. https://doi.org/10.1503/cmaj.190211
Schroeder MC, Chapman CG, Nattinger MC et al (2016) Variation in geographic access to chemotherapy by definitions of providers and service locations: a population-based observational study. BMC Health Serv Res 16:274. https://doi.org/10.1186/s12913-016-1549-5
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36(1):8–27. https://doi.org/10.1097/00005650-199801000-00004
Kralji B (2000) Measuring, “rurality” for purposes of health-care planning: an empirical measure for Ontario. Ont Med Rev 67:33–52
Health Quality Ontario. Geographic Location Methods Review: Summary Report. https://www.hqontario.ca/Portals/0/documents/pr/hqo-geographic-location-methods-review-report.pdf.
Matheson FI, Moloney G, van Inge T. 2016 Ontario Marginalization Index User Guide.
Tøge AG, Bell R (2016) Material deprivation and health: a longitudinal study. BMC Public Health 16:747. https://doi.org/10.1186/s12889-016-3327-z
Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A (2010) AJCC Cancer Staging Manual. 7th ed. Springer.
Hortobagyi G, Connolly J. AJCC Cancer Staging Manual (2016) 8th ed. (Amin M, Edge S, Greene F, et al, eds.) Springer International Publisher.
Cancer Care Ontario: Regional Cancer Centres https. https://www.cancercareontario.ca/en/find-cancer-services/regional-cancer-centres/list.
Austin PC (2009) Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38(6):1228–1234. https://doi.org/10.1080/03610910902859574
Ballinger GA (2004) Using generalized estimating equations for longitudinal data analysis. Organ Res Methods 7(2):127–150. https://doi.org/10.1177/1094428104263672
Riedel F, Hoffmann AS, Moderow M et al (2020) Time trends of neoadjuvant chemotherapy for early breast cancer. Int J Cancer 147(11):3049–3058. https://doi.org/10.1002/ijc.33122
Powis M, Groome P, Biswanger N et al (2019) Cross-Canada differences in early-stage breast cancer treatment and acute-care use. Curr Oncol 26(5):624–639. https://doi.org/10.3747/co.26.5003
Yee EK, Coburn NG, Zuk V et al (2021) Geographic impact on access to care and survival for non-curative esophagogastric cancer: a population-based study. Gastric Cancer 24(4):790–799. https://doi.org/10.1007/s10120-021-01157-w
Yee EK, Coburn NG, Davis LE et al (2020) Impact of geography on care delivery and survival for noncurable pancreatic adenocarcinoma: a population-based analysis. J Natl Compr Canc Netw 18(12):1642–1650. https://doi.org/10.6004/jnccn.2020.7605
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from Canadian Cancer Society Challenge Grant. This study was supported by the Sunnybrook AFP Association through the Innovation Fund of the Alternative Funding Plan from the Academic Health Sciences Centres of Ontario. Parts of this material are based on data and/or information compiled and provided by Cancer Care Ontario (CCO). The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lena Nguyen. The first draft of the manuscript was written by Amanda Roberts and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roberts, A., Hallet, J., Nguyen, L. et al. Neoadjuvant chemotherapy for triple-negative and Her2 +ve breast cancer: striving for the standard of care. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07282-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07282-1