Abstract
Purpose
Chemotherapy-induced cardiotoxicity is a critical issue for patients with breast cancer. Change of epicardial adipose tissue (EAT) is associated with cardiac dysfunction. The objective of this study was to investigate the relationship between EAT and chemotherapy-induced cardiotoxicity.
Methods
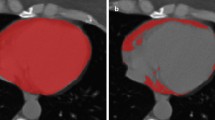
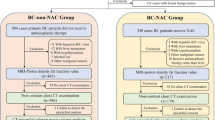
This retrospective study analyzed EAT on chest computed tomography (CT) of patients with early breast cancer using automatic, quantitative measurement software between November 2015 and January 2020. Changes in EAT before and after initiation of chemotherapy were compared according to the type of anticancer drug. Subclinical cardiotoxicity was defined as worsening ≥ 10% in left ventricular ejection fraction to an absolute value > 50% with a lower limit of normal measured with standard echocardiography.
Results
Among 234 patients with breast cancer, 85 were treated with adjuvant anthracycline-based (AC) and 149 were treated with non-anthracycline-based (non-AC) chemotherapy. There was a significant increase in EAT volume index (mL/kg/m2) at the end of chemotherapy compared to that at the baseline in the AC group (3.33 ± 1.53 vs. 2.90 ± 1.52, p < 0.001), but not in the non-AC group. During the follow-up period, subclinical cardiotoxicity developed in 20/234 (8.6%) patients in the total population [13/85 (15.3%) in the AC group and 7/149 (4.8%) in the non-AC group]. In the multivariable analysis, EAT volume index increment after chemotherapy was associated with a lower risk of subclinical cardiotoxicity in the AC group (Odds ratio: 0.364, 95% CI 0.136–0.971, p = 0.044).
Conclusions
Measurement of EAT during anthracycline-based chemotherapy might help identify subgroups who are vulnerable to chemotherapy-induced cardiotoxicity. Early detection of EAT volume change could enable tailored chemotherapy with cardiotoxicity prevention strategies.



Similar content being viewed by others
Data availability
The data underlying this article cannot be shared publicly to protect the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
References
Choi JY et al (2018) Incidence and risk factors for congestive heart failure in patients with early breast cancer who received anthracycline and/or trastuzumab: a big data analysis of the Korean Health Insurance Review and Assessment service database. Breast Cancer Res Treat 171(1):181–188
Henriksen PA (2018) Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104(12):971–977
Heck SL et al (2021) Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow-up of a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation 143(25):2431–2440
Iacobellis G (2015) Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11(6):363–371
Mahabadi AA et al (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf recall study. J Am Coll Cardiol 61(13):1388–1395
Monti CB et al (2021) Potential role of epicardial adipose tissue as a biomarker of anthracycline cardiotoxicity. Insights Imaging 12(1):161
Seidman A et al (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20(5):1215–1221
Arangalage D et al (2019) Epicardial adipose tissue volume is associated with left ventricular remodelling in calcific aortic valve stenosis. Arch Cardiovasc Dis 112(10):594–603
World Health Organization Regional Office for the Western Pacific, The Asia-Pacific perspective: redefining obesity and its treatment. 2000, Sydney, Australia: Health Communications Australia
Oikonomou EK et al (2018) Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 392(10151):929–939
Okayama S et al (2012) The influence of effective energy on computed tomography number depends on tissue characteristics in monoenergetic cardiac imaging. Radiol Res Pract 2012:150980
Nagayama Y et al (2019) Epicardial fat volume measured on nongated chest CT is a predictor of coronary artery disease. Eur Radiol 29(7):3638–3646
Dobson R et al (2021) BSE and BCOS guideline for transthoracic echocardiographic assessment of adult cancer patients receiving anthracyclines and/or trastuzumab. JACC CardioOncol 3(1):1–16
Lang RM et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28(1):1-39.e14
Henderson IC et al (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21(6):976–983
Kim H et al (2018) Diagnosis, treatment, and prevention of cardiovascular toxicity related to anti-cancer treatment in clinical practice: an opinion paper from the working group on cardio-oncology of the korean society of echocardiography. J Cardiovasc Ultrasound 26(1):1–25
Sarin S et al (2008) Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol 102(6):767–771
Doesch C et al (2010) Bioimpedance analysis parameters and epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure. Obesity (Silver Spring) 18(12):2326–2332
Iacobellis G et al (2014) Epicardial fat in atrial fibrillation and heart failure. Horm Metab Res 46(8):587–590
Pugliese, NR, et al (2021) Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 23(11):1858-1871
Packer M (2018) Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation 137(15):1614–1631
Sacks HS et al (2009) Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 94(9):3611–3615
Baba S et al (2010) CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med 51(2):246–250
Antonopoulos AS et al (2017) Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 9:398
Walker J et al (2010) Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol 28(21):3429–3436
Thavendiranathan P et al (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63(25):2751–2768
Acknowledgements
This research was partly supported by the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (NRF-2022R1A2C1007571, NRF-2022R1G1A1009823), and the Soonchunhyang University research fund.
Author information
Authors and Affiliations
Contributions
SHK contributed to conceptualization and methodology. SSK contributed to formal analysis, visualization, and writing—original draft. BDN contributed to formal analysis and data curation. M-YL, MHL, B-WP, and DWB contributed to writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwon, S.S., Nam, B.D., Lee, MY. et al. Increased EAT volume after anthracycline chemotherapy is associated with a low risk of cardiotoxicity in breast cancer. Breast Cancer Res Treat 196, 111–119 (2022). https://doi.org/10.1007/s10549-022-06696-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06696-z