Abstract
Objectives
To investigate the prevalence of chemotherapy-associated steatohepatitis, quantitate the epicardial adipose tissue (EAT) volume in breast cancer patients, and explore the mediating effect of liver fat content on EAT volume in breast cancer patients who received neoadjuvant chemotherapy (NAC).
Methods
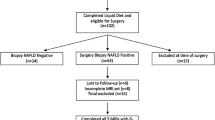
From October 2018 to April 2020, patients were retrospectively reviewed and divided into breast cancer non-NAC and NAC groups. The prevalence of chemotherapy-associated steatohepatitis was evaluated through quantitative MRI mDIXON-Quant examinations by using defined proton density fat fraction cutoffs of liver fat. The EAT volume was quantified on chest CT by semi-automatic volume analysis software. Bootstrap analysis was used in the breast cancer NAC group to test the significance of the mediating effect of liver fat content on EAT volume.
Results
A total of 662 breast cancer patients (non-NAC group: 445 patients; NAC group: 217 patients) were included. The prevalence of chemotherapy-associated steatohepatitis in the NAC group was significantly higher than the prevalence of hepatic steatosis in the non-NAC group (42.8% vs. 33.3%, p < 0.001). EAT volume was measured in 561 of 662 breast cancer patients, and was significantly higher in the NAC group than in the non-NAC group (137.26 ± 53.48 mL vs. 125.14 ± 58.77 mL, p = 0.020). In the breast cancer NAC group, the indirect effect of liver fat content on EAT volume was 2.545 (p < 0.001), and the contribution rate to the effect was 69.1%.
Conclusions
EAT volume was significantly higher in the BC-NAC group than in the BC-non-NAC group.
Key Points
• The prevalence of CASH was as high as 42.8% in BC patients.
• NAC significantly increased the EAT volume in BC patients.
• The liver fat content caused the change of EAT volume through mediating effect.






Similar content being viewed by others
Abbreviations
- BC:
-
Breast cancer
- BMI:
-
Body mass index
- CASH:
-
Chemotherapy-associated steatohepatitis
- CVD:
-
Cardiovascular disease
- EAT:
-
Epicardial adipose tissue
- ICC:
-
Intraclass correlation coefficient
- MRI:
-
Magnetic resonance imaging
- NAC:
-
Neoadjuvant chemotherapy
- NAFLD:
-
Nonalcoholic fatty liver disease
- PDFF:
-
Proton density fat fraction
- ROI:
-
Region of interest
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Derks MGM, van de Velde CJH (2018) Neoadjuvant chemotherapy in breast cancer: more than just downsizing. Lancet Oncol 19:2–3
Gradishar WJ, Anderson BO, Balassanian R et al (2018) Breast cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 16:310–320
Banke A, Fosbol EL, Ewertz M et al (2019) Long-term risk of heart failure in breast cancer patients after adjuvant chemotherapy with or without trastuzumab. JACC Heart Fail 7:217–224
Matos E, Jug B, Blagus R, Zakotnik B (2016) A prospective cohort study on cardiotoxicity of adjuvant trastuzumab therapy in breast cancer patients. Arq Bras Cardiol 107:40–47
Schumacher JD, Guo GL (2015) Mechanistic review of drug-induced steatohepatitis. Toxicol Appl Pharmacol 289:40–47
Dash A, Figler RA, Sanyal AJ, Wamhoff BR (2017) Drug-induced steatohepatitis. Expert Opin Drug Metab Toxicol 13:193–204
Meunier L, Larrey D (2020) Chemotherapy-associated steatohepatitis. Ann Hepatol 19:597–601
Vigano L, De Rosa G, Toso C et al (2017) Reversibility of chemotherapy-related liver injury. J Hepatol 67:84–91
Inci F, Karatas F (2019) Paclitaxel-induced hepatic steatosis in patients with breast cancer. J BUON 24:2355–2360
Castera L, Friedrich-Rust M, Loomba R (2019) Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156(1264-1281):e1264
Hui SCN, So HK, Chan DFY et al (2018) Validation of water-fat MRI and proton MRS in assessment of hepatic fat and the heterogeneous distribution of hepatic fat and iron in subjects with non-alcoholic fatty liver disease. Eur J Radiol 107:7–13
Yokoo T, Shiehmorteza M, Hamilton G et al (2011) Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 258:749–759
Meisamy S, Hines CD, Hamilton G et al (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258:767–775
Chaosuwannakit N, D’Agostino R Jr, Hamilton CA et al (2010) Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol 28:166–172
Koelwyn GJ, Lewis NC, Ellard SL et al (2016) Ventricular-arterial coupling in breast cancer patients after treatment with anthracycline-containing adjuvant chemotherapy. Oncologist 21:141–149
Zhang W, Xie K, Fu S et al (2019) Comparison of the incidence of perioperative cardiovascular risk events among patients with and without a history of neoadjuvant chemotherapy. Minerva Anestesiol 85:822–829
Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH (2017) Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 2. J Am Coll Cardiol 70:2552–2565
Zeng X, Wang X, Chen H et al (2020) Evaluating the image quality of monoenergetic images from dual-energy computed tomography with low-concentration and low-flow-rate contrast media for the arterials supply to the nipple-areola complex in breast cancer compared with conventional computed tomography angiography. J Comput Assist Tomogr 44:921–927
Eisenberg E, McElhinney PA, Commandeur F et al (2020) Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging 13:e009829
Russo R, Di Iorio B, Di Lullo L, Russo D (2018) Epicardial adipose tissue: new parameter for cardiovascular risk assessment in high risk populations. J Nephrol 31:847–853
Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE (2020) Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 51:728–736
Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H (2020) Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res 126:679–704
Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G (2014) Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol 20:1724–1745
Younossi Z, Anstee QM, Marietti M et al (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15:11–20
Tana C, Ballestri S, Ricci F et al (2019) Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health 16
Kuhn JP, Meffert P, Heske C et al (2017) Prevalence of fatty liver disease and hepatic iron overload in a northeastern German population by using quantitative MR imaging. Radiology 284:706–716
Kuhn JP, Hernando D, Munoz del Rio A et al (2012) Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology 265:133–142
Kwon EY, Kim YR, Kang DM, Yoon KH, Lee YH (2021) Usefulness of US attenuation imaging for the detection and severity grading of hepatic steatosis in routine abdominal ultrasonography. Clin Imaging 76:53–59
Gaubeta S, Klinghammer L, Jahn D, Schuhback A, Achenbach S, Marwan M (2014) Epicardial fat and coronary artery calcification in patients on long-term hemodialysis. J Comput Assist Tomogr 38:768–772
Hell MM, Achenbach S, Schuhbaeck A, Klinghammer L, May MS, Marwan M (2016) CT-based analysis of pericoronary adipose tissue density: relation to cardiovascular risk factors and epicardial adipose tissue volume. J Cardiovasc Comput Tomogr 10:52–60
Marwan M, Koenig S, Schreiber K et al (2019) Quantification of epicardial adipose tissue by cardiac CT: influence of acquisition parameters and contrast enhancement. Eur J Radiol 121:108732
Elsanhoury A, Nelki V, Kelle S, Van Linthout S, Tschope C (2021) Epicardial fat expansion in diabetic and obese patients with heart failure and preserved ejection fraction-a specific HFpEF phenotype. Front Cardiovasc Med 8:720690
Artz NS, Haufe WM, Hooker CA et al (2015) Reproducibility of MR-based liver fat quantification across field strength: same-day comparison between 1.5T and 3T in obese subjects. J Magn Reson Imaging 42:811–817
Serai SD, Dillman JR, Trout AT (2017) Proton density fat fraction measurements at 1.5- and 3-T hepatic MR imaging: same-day agreement among readers and across two imager manufacturers. Radiology 284:244–254
Tang A, Tan J, Sun M et al (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267:422–431
Jayakumar S, Middleton MS, Lawitz EJ et al (2019) Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol 70:133–141
Labbe G, Pessayre D, Fromenty B (2008) Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol 22:335–353
Lancellotti P, Anker SD, Donal E et al (2015) EACVI/HFA Cardiac Oncology Toxicity Registry in breast cancer patients: rationale, study design, and methodology (EACVI/HFA COT Registry)--EURObservational Research Program of the European Society of Cardiology. Eur Heart J Cardiovasc Imaging 16:466–470
Madonna R, Massaro M, Scoditti E, Pescetelli I, De Caterina R (2019) The epicardial adipose tissue and the coronary arteries: dangerous liaisons. Cardiovasc Res 115:1013–1025
Packer M (2018) Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 71:2360–2372
Christensen RH, von Scholten BJ, Hansen CS et al (2019) Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol 18:114
Adams LA, Anstee QM, Tilg H, Targher G (2017) Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66:1138–1153
van den Berg EH, Wolters AAB, Dullaart RPF et al (2019) Prescription of statins in suspected non-alcoholic fatty liver disease and high cardiovascular risk, a population-based study. Liver Int 39:1343–1354
Xia LY, Hu QL, Zhang J, Xu WY, Li XS (2020) Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: a meta-analysis of 36,480 cases. World J Surg Oncol 18:129
Idilman IS, Aniktar H, Idilman R et al (2013) Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 267:767–775
Acknowledgements
We thank the study participants and referring technicians for their participation in this study. We acknowledge the support of Xiaoyue Zhang from Siemens scientific research.
Funding
This study has received funding by the Natural Science Foundation of Chongqing municipality (cstc2021jcyj-msxmX0387), the Medical Scientific Research Project of Chongqing Municipal Health Commission (2022WSJK027), and the 2021 SKY Imaging Research Fund of the Chinese International Medical Foundation (Z-2014-07-2101).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jiuquan Zhang, from the Department of Radiology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, People’s Republic of China (zhangjq_radiol@foxmail.com).
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Tan, Y., Liu, D. et al. Chemotherapy-associated steatohepatitis was concomitant with epicardial adipose tissue volume increasing in breast cancer patients who received neoadjuvant chemotherapy. Eur Radiol 32, 4898–4908 (2022). https://doi.org/10.1007/s00330-022-08581-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08581-1