Abstract
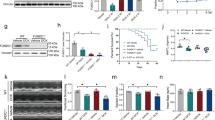
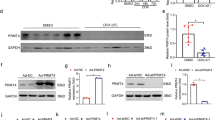
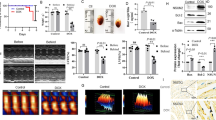
Doxorubicin (DOX) is an anthracycline antibiotic used as an antitumor treatment. However, its clinical application is limited due to severe side effects such as cardiotoxicity. In recent years, numerous studies have demonstrated that cellular aging has become a therapeutic target for DOX-induced cardiomyopathy. However, the underlying mechanism and specific molecular targets of DOX-induced cardiomyocyte aging remain unclear. Poly (ADP-ribose) polymerase (PARP) is a family of protein post-translational modification enzymes in eukaryotic cells, including 18 members. PARP-1, the most well-studied member of this family, has become a potential molecular target for the prevention and treatment of various cardiovascular diseases, such as DOX cardiomyopathy and heart failure. PARP-1 and PARP-2 share 69% homology in the catalytic regions. However, they do not entirely overlap in function. The role of PARP-2 in cardiovascular diseases, especially in DOX-induced cardiomyocyte aging, is less studied. In this study, we found for the first time that down-regulation of PARP-2 can inhibit DOX-induced cellular aging in cardiomyocytes. On the contrary, overexpression of PARP-2 can aggravate DOX-induced cardiomyocyte aging and injury. Further research showed that PARP-2 inhibited the expression and activity of SIRT1, which in turn was involved in the development of DOX-induced cardiomyocyte aging and injury. Our findings provide a preliminary experimental basis for establishing PARP-2 as a new target for preventing and treating DOX cardiomyopathy and related drug development.











Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Abbreviations
- DOX:
-
Doxorubicin
- PARPs:
-
Poly (ADP-ribose) polymerases
- DMEM:
-
Dulbecco’s modified eagle medium
- FBS:
-
Fetal bovine serum
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- DCFH-DA:
-
2,7-Dichlorodihydrofluorescein diacetate
- DAMPs:
-
Damage-associated molecular patterns
- HE:
-
Hematoxylin-eosin
- CCK-8:
-
Cell counting kit-8
- γ-H2AX:
-
Phosphorylated histone H2AX
- IVSd:
-
Interventricular septal thickness during diastole
- IVSs:
-
Interventricular septal thickness during systole
- LVIDd:
-
Left ventricular internal diameter during diastole
- LVIDs:
-
Left ventricular internal diameter during systole
- LVPWd:
-
Left ventricular posterior wall thickness during diastole
- LVPWs:
-
Left ventricular posterior wall thickness during systole
References
Sawicki KT, Sala V, Prever L et al (2021) Preventing and treating anthracycline cardiotoxicity: new insights. Annu Rev Pharmacol Toxicol 61:309–332
Ahmad N, Ullah A, Chu P et al (2022) Doxorubicin induced cardio toxicity through sirtuins mediated mitochondrial disruption. Chem Biol Interact 365:110028
Steinherz LJ, Steinherz PG, Tan CT et al (1991) Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266:1672–1677
Felker GM, Thompson RE, Hare JM et al (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342:1077–1084
Kumari H, Huang WH, Chan MWY (2020) Review on the role of epigenetic modifications in doxorubicin-induced cardiotoxicity. Front Cardiovasc Med 7:56
Songbo M, Lang H, Xinyong C et al (2019) Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett 307:41–48
Li N, Wang Y, Deng W et al (2019) Poly (ADP-ribose) polymerases (PARPs) and PARP inhibitor-targeted therapeutics. Anticancer Agents Med Chem 19:206–212
Przybycinski J, Nalewajska M, Marchelek-Mysliwiec M et al (2019) Poly-ADP-ribose polymerases (PARPs) as a therapeutic target in the treatment of selected cancers. Expert Opin Ther Targets 23:773–785
Barreiro E, Gea J (2018) PARP-1 and PARP-2 activity in cancer-induced cachexia: potential therapeutic implications. Biol Chem 399:179–186
Pacher P, Liaudet L, Bai P et al (2002) Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther 300:862–867
Wang H, Yang X, Yang Q et al (2018) PARP-1 inhibition attenuates cardiac fibrosis induced by myocardial infarction through regulating autophagy. Biochem Biophys Res Commun 503:1625–1632
Yeo D, Kang C, Ji LL (2020) Aging alters acetylation status in skeletal and cardiac muscles. Geroscience 42:963–976
Ame JC, Rolli V, Schreiber V et al (1999) PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem 274:17860–17868
Szanto M, Rutkai I, Hegedus C et al (2011) Poly(ADP-ribose) polymerase-2 depletion reduces doxorubicin-induced damage through SIRT1 induction. Cardiovasc Res 92:430–438
Almeida M, Porter RM (2019) Sirtuins and FoxOs in osteoporosis and osteoarthritis. Bone 121:284–292
Minor RK, Baur JA, Gomes AP et al (2011) SRT1720 improves survival and healthspan of obese mice. Sci Rep 1:70
Mitchell SJ, Martin-Montalvo A, Mercken EM et al (2014) The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6:836–843
Donato AJ, Magerko KA, Lawson BR et al (2011) SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589:4545–4554
D’Onofrio N, Servillo L, Giovane A et al (2016) Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: involvement of SIRT1 and SIRT6. Free Radic Biol Med 96:211–222
Kitada M, Ogura Y, Koya D (2016) The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging 8:2290–2307
Satoh A, Brace CS, Rensing N et al (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18:416–430
Lim CJ, Lee YM, Kang SG et al (2017) Aquatide activation of SIRT1 reduces cellular senescence through a SIRT1-FOXO1-autophagy axis. Biomol Ther (Seoul) 25:511–518
Bai P, Canto C, Brunyanszki A et al (2011) PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab 13:450–460
Cai Y, Yu SS, Chen SR et al (2012) Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett 586:866–874
Zhang T, Zhang Y, Cui M et al (2016) CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress–induced myocardial necroptosis. Nat Med 22:175–182
Di Micco R, Krizhanovsky V, Baker D et al (2021) Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 22:75–95
Campisi J (2013) Aging, cellular senescence, and cancer. Annu Rev Physiol 75:685–705
Papaconstantinou J (2019) The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells 8:1383
Kelleher AM, Setlem R, Dantzer F et al (2021) Deficiency of PARP-1 and PARP-2 in the mouse uterus results in decidualization failure and pregnancy loss. Proc Natl Acad Sci USA 118:e2109252118
Yelamos J, Moreno-Lama L, Jimeno J et al (2020) Immunomodulatory roles of PARP-1 and PARP-2: impact on PARP-centered cancer therapies. Cancers (Basel) 12:392
Sodhi RK, Singh N, Jaggi AS (2010) Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol 53:77–87
Kanagasabai R, Karthikeyan K, Zweier JL et al (2021) Serine mutations in overexpressed Hsp27 abrogate the protection against doxorubicin-induced p53-dependent cardiac apoptosis in mice. Am J Physiol Heart Circ Physiol 321:H963-h975
Naka KK, Vezyraki P, Kalaitzakis A et al (2014) Hsp70 regulates the doxorubicin-mediated heart failure in Hsp70-transgenic mice. Cell Stress Chaperones 19:853–864
Damiani RM, Moura DJ, Viau CM et al (2018) Influence of PARP-1 inhibition in the cardiotoxicity of the topoisomerase 2 inhibitors doxorubicin and mitoxantrone. Toxicol in Vitro 52:203–213
Riccio AA, Cingolani G, Pascal JM (2016) PARP-2 domain requirements for DNA damage-dependent activation and localization to sites of DNA damage. Nucleic Acids Res 44:1691–1702
North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110:1097–1108
Martin-Fernandez B, Gredilla R (2016) Mitochondria and oxidative stress in heart aging. Age (Dordr) 38:225–238
Bou-Teen D, Kaludercic N, Weissman D et al (2021) Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic Biol Med 167:109–124
Reuter S, Gupta SC, Chaturvedi MM et al (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Uyar B, Palmer D, Kowald A et al (2020) Single-cell analyses of aging, inflammation and senescence. Ageing Res Rev 64:101156
Koleini N, Nickel BE, Edel AL et al (2019) Oxidized phospholipids in doxorubicin-induced cardiotoxicity. Chem Biol Interact 303:35–39
Yarmohammadi F, Karbasforooshan H, Hayes AW et al (2021) Inflammation suppression in doxorubicin-induced cardiotoxicity: natural compounds as therapeutic options. Naunyn Schmiedebergs Arch Pharmacol 394:2003–2011
Li D, Yang Y, Wang S et al (2021) Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol 46:102089
Beneke S, Burkle A (2004) Poly(ADP-ribosyl)ation, PARP, and aging. Sci Aging Knowledge Environ 2004:re9
Zhao L, Cao J, Hu K et al (2020) Sirtuins and their biological relevance in aging and age-related diseases. Aging Dis 11:927–945
Fang EF, Scheibye-Knudsen M, Brace LE et al (2014) Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157:882–896
Murtaza G, Khan AK, Rashid R et al (2017) FOXO transcriptional factors and long-term living. Oxid Med Cell Longev 2017:3494289
Kobayashi Y, Furukawa-Hibi Y, Chen C et al (2005) SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med 16:237–243
Huang K, Yan ZQ, Zhao D et al (2015) SIRT1 and FOXO mediate contractile differentiation of vascular smooth muscle cells under cyclic Stretch. Cell Physiol Biochem 37:1817–1829
Firat E, Niedermann G (2016) FoxO proteins or loss of functional p53 maintain stemness of glioblastoma stem cells and survival after ionizing radiation plus PI3K/mTOR inhibition. Oncotarget 7:54883–54896
Li J, Wang PY, Long NA et al (2019) p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities. Proc Natl Acad Sci U S A 116:19626–19634
Funding
This research was supported by grants from the Guangzhou Basic and Applied Basic Research Project (No. 202102020199), the Medical Science and Technology research Foundation of Guangdong Province (No. A2022063), the Guangzhou Medical University Discipline Construction Project-Epigenetic Drug R&D Incubation Program (No. 02-410-2206311), the College Students’ Science and Technology Innovation Project of Guangzhou Medical University (No. 2021A073), Guangzhou Medical University Discipline Construction Project-Pharmacy Top Talent Training Undergraduate Program (No. 02-410-2206302, No. 02-410-2206296 ), and the open research from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (No. 202201-205).
Author information
Authors and Affiliations
Contributions
Conception or design of the work: CH, XZ and YC; Data collection: SW, AS, SG, TX, YH, YX, YZ, JC, RL; Drafting the article and critical revision of the article: YZ, CW, YC; Final ap-proval of the version to be published: All authors and All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Ethical approval
All animal studies were approved by the Institutional Animal Care and Use Committee at Guangzhou Medical University (Protocol GY2020–059).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, C., Zhang, X., Wang, S. et al. PARP-2 mediates cardiomyocyte aging and damage induced by doxorubicin through SIRT1 Inhibition. Apoptosis 29, 816–834 (2024). https://doi.org/10.1007/s10495-023-01929-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01929-y