Abstract
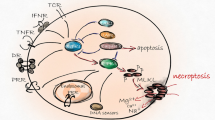
Necroptosis is a type of programmed cell death that is morphologically similar to necrosis. This type of cell death is involved in various pathophysiological disorders, including inflammatory, neurodegenerative, infectious, and malignant diseases. Receptor-interacting protein kinase 1 (RIPK1), RIPK3, and mixed lineage kinase domain-like protein (MLKL) pseudokinase constitute the core components of the necroptosis signaling pathway and are considered the most promising targets for therapeutic intervention. The discovery and characterization of necroptosis inhibitors not only accelerate our understanding of the necroptosis signaling pathway but also provide important drug candidates for the treatment of necroptosis-related diseases. Here, we will review recent research progress on necroptosis inhibitors, mechanisms of action and their potential applications for disease treatment.





















Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- PCD:
-
Programmed cell death
- RIPK1:
-
Receptor-interacting protein kinase 1
- RIPK3:
-
Receptor-interacting protein kinase 3
- MLKL:
-
Mixed lineage kinase domain-like protein
- TNF:
-
Tumor necrosis factor
- TLR:
-
Toll-like receptor
- IFNAR:
-
Interferon receptors
- ZBP1:
-
Z-DNA binding protein 1
- TNFR1:
-
TNF receptor 1
- TRADD:
-
TNFR1-associated death domain protein
- cIAP1/2:
-
Cellular inhibitor of apoptosis proteins 1 and 2
- TRAF2/5:
-
Tumor necrosis factor receptor-associated factor 2/5
- TAK1:
-
Transforming growth factor-β-activated kinase 1
- IKK:
-
IκB kinase
- SMAC:
-
Second mitochondria-derived activator of caspase
- CYLD:
-
Cylindromatosis
- FADD:
-
Fas-associated death domain protein
- RHIM:
-
RIP homotypic interaction motif
- TRIF:
-
TIR-domain-containing adapter-inducing interferon-β
- Nec-1:
-
Necrostatin-1
- IDO:
-
Indoleamine 2,3-dioxygenase
- AKI:
-
Acute kidney injury
- AAA:
-
Abdominal aortic aneurysm
- PDGFRα:
-
Platelet-derived growth factor receptor α
- SIRS:
-
Systemic inflammatory response syndrome
- PERK:
-
Protein kinase R-like ER kinase
- AurK:
-
Pan-Aurora kinase
- IECs:
-
Intestinal epithelial cells
- PK:
-
Pharmacokinetic
- EAE:
-
Experimental autoimmune encephalomyelitis
- MS:
-
Multiple sclerosis
- IMIDs:
-
Inflammatory illnesses
- CNS:
-
Central nervous system
- AD:
-
Alzheimer disease
- ALS:
-
Amyotrophic lateral sclerosis
- FIH:
-
First-in-human
- IC50:
-
Half maximal inhibitory concentration
- LDH:
-
Lactate dehydrogenase
- mPTP:
-
Mitochondrial permeability transition pore
- H/R:
-
Hypoxia/reoxygenation
- NSA:
-
Necrosulfonamide
- SAR:
-
Structure–activity relationship
- HSP90:
-
Heat shock protein 90
- GA:
-
Geldanamycin
- 17AAG:
-
17-Allylamino-17-desmethoxygeldanamycin
- KA:
-
Kongensin A
- 6,7-DHC:
-
6,7-Dihydroxycoumarin
- CKD:
-
Chronic kidney disease
- PKIS:
-
Published kinase inhibitor set
- FN3:
-
Fibronectin type III
- 4HB:
-
Four-helix bundle
- NBC1:
-
Necroptosis-blocking compound 1
- PROTAC:
-
Proteolysis targeting chimeric technology
References
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147(4):742–758
Weinlich R, Oberst A, Beere HM et al (2017) Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18(2):127–136
Wallach D, Kang TB, Dillon CP et al (2016) Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352(6281):aaf2154
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517(7534):311–320
Upton JW, Kaiser WJ, Mocarski ES (2019) DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 26(4):564
Cho YS, Challa S, Moquin D et al (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123
He S, Wang L, Miao L et al (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137(6):1100–1111
Sun L, Wang H, Wang Z et al (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148(1–2):213–227
Zhao J, Jitkaew S, Cai Z et al (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA 109(14):5322–5327
Park J, Ha HJ, Chung ES et al (2021) O-GlcNAcylation ameliorates the pathological manifestations of Alzheimer’s disease by inhibiting necroptosis. Sci Adv. https://doi.org/10.1126/sciadv.abd3207
Wang T, Perera ND, Chiam MDF et al (2020) Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ 27(5):1728–1739
Strilic B, Yang L, Albarran-Juarez J et al (2016) Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536(7615):215–218
Jiao D, Cai Z, Choksi S et al (2018) Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res 28(8):868–870
Ye K, Chen Z, Xu Y (2023) The double-edged functions of necroptosis. Cell Death Dis 14(2):163
Vandenabeele P, Galluzzi L, Vanden Berghe T et al (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11(10):700–714
Chen Y, Ren W, Wang Q et al (2022) The regulation of necroptosis by ubiquitylation. Apoptosis 27(9–10):668–684
Chen G, Goeddel DV (2002) TNF-R1 signaling: a beautiful pathway. Science 296(5573):1634–1635
Moquin DM, McQuade T, Chan FK (2013) CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE 8(10):e76841
Fritsch M, Gunther SD, Schwarzer R et al (2019) Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575(7784):683–687
Newton K, Wickliffe KE, Dugger DL et al (2019) Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574(7778):428–431
Cai Z, Jitkaew S, Zhao J et al (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16(1):55–65
Chen X, Li W, Ren J et al (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24(1):105–121
Dondelinger Y, Declercq W, Montessuit S et al (2014) MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7(4):971–981
Wang H, Sun L, Su L et al (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54(1):133–146
He S, Liang Y, Shao F et al (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA 108(50):20054–20059
Kaiser WJ, Sridharan H, Huang C et al (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288(43):31268–31279
Kuriakose T, Kanneganti TD (2018) ZBP1: innate sensor regulating cell death and inflammation. Trends Immunol 39(2):123–134
Zheng M, Kanneganti TD (2020) The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev 297(1):26–38
Muller S, Chaikuad A, Gray NS et al (2015) The ins and outs of selective kinase inhibitor development. Nat Chem Biol 11(11):818–821
Mobitz H (2015) The ABC of protein kinase conformations. Biochim Biophys Acta 1854(10 Pt B):1555–1566
Degterev A, Huang Z, Boyce M et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119
Degterev A, Hitomi J, Germscheid M et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4(5):313–321
Xie T, Peng W, Liu Y et al (2013) Structural basis of RIP1 inhibition by necrostatins. Structure 21(3):493–499
Cao L, Mu W (2021) Necrostatin-1 and necroptosis inhibition: pathophysiology and therapeutic implications. Pharmacol Res 163:105297
Takahashi N, Duprez L, Grootjans S et al (2012) Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis 3(11):e437
Zhuang C, Chen F (2020) Small-molecule inhibitors of necroptosis: current status and perspectives. J Med Chem 63(4):1490–1510
Martin-Sanchez D, Fontecha-Barriuso M, Carrasco S et al (2018) TWEAK and RIPK1 mediate a second wave of cell death during AKI. Proc Natl Acad Sci USA 115(16):4182–4187
Wang Q, Zhou T, Liu Z et al (2017) Inhibition of receptor-interacting protein kinase 1 with necrostatin-1s ameliorates disease progression in elastase-induced mouse abdominal aortic aneurysm model. Sci Rep 7:42159
Jhun J, Lee SH, Kim SY et al (2019) RIPK1 inhibition attenuates experimental autoimmune arthritis via suppression of osteoclastogenesis. J Transl Med 17(1):84
Thomas CN, Thompson AM, Ahmed Z et al (2019) Retinal ganglion cells die by necroptotic mechanisms in a site-specific manner in a rat blunt ocular injury model. Cells 8(12):1517
Ma XR, Yang SY, Zheng SS et al (2022) Inhibition of RIPK1 by ZJU-37 promotes oligodendrocyte progenitor proliferation and remyelination via NF-kappaB pathway. Cell Death Discov 8(1):147
Niu A, Lin L, Zhang D et al (2022) Discovery of novel 2,8-diazaspiro[4.5]decan-1-one derivatives as potent RIPK1 kinase inhibitors. Bioorg Med Chem 59:116686
Rojas-Rivera D, Delvaeye T, Roelandt R et al (2017) When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ 24(6):1100–1110
Harris PA, Bandyopadhyay D, Berger SB et al (2013) Discovery of small molecule RIP1 kinase inhibitors for the treatment of pathologies associated with necroptosis. ACS Med Chem Lett 4(12):1238–1243
Fauster A, Rebsamen M, Huber KV et al (2015) A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis 6:e1767
Najjar M, Suebsuwong C, Ray SS et al (2015) Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1. Cell Rep 10(11):1850–1860
Rui C, Shi SN, Ren W et al (2021) The multitargeted kinase inhibitor KW-2449 ameliorates cisplatin-induced nephrotoxicity by targeting RIPK1-mediated necroptosis. Biochem Pharmacol 188:114542
Qin X, Hu L, Shi SN et al (2020) The Bcr-Abl inhibitor GNF-7 inhibits necroptosis and ameliorates acute kidney injury by targeting RIPK1 and RIPK3 kinases. Biochem Pharmacol 177:113947
Martens S, Goossens V, Devisscher L et al (2018) RIPK1-dependent cell death: a novel target of the Aurora kinase inhibitor tozasertib (VX-680). Cell Death Dis 9(2):211
Hofmans S, Devisscher L, Martens S et al (2018) Tozasertib analogues as inhibitors of necroptotic cell death. J Med Chem 61(5):1895–1920
Wang JN, Liu MM, Wang F et al (2019) RIPK1 inhibitor Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via attenuating necroptosis, inflammation and oxidative stress. Clin Sci (Lond) 133(14):1609–1627
Harris PA, King BW, Bandyopadhyay D et al (2016) DNA-encoded library screening identifies benzo[b][1,4]oxazepin-4-ones as highly potent and monoselective receptor interacting protein 1 kinase inhibitors. J Med Chem 59(5):2163–2178
Xia C, Yao Z, Xu L et al (2021) Structure-based bioisosterism design of thio-benzoxazepinones as novel necroptosis inhibitors. Eur J Med Chem 220:113484
Harris PA, Berger SB, Jeong JU et al (2017) Discovery of a first-in-class receptor interacting protein 1 (RIP1) kinase specific clinical candidate (GSK2982772) for the treatment of inflammatory diseases. J Med Chem 60(4):1247–1261
Weisel K, Scott NE, Tompson DJ et al (2017) Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol Res Perspect. https://doi.org/10.1002/prp2.365
Tompson D, Whitaker M, Pan R et al (2022) Development of a once-daily modified-release formulation for the short half-life RIPK1 inhibitor GSK2982772 using DiffCORE technology. Pharm Res 39(1):153–165
Weisel K, Scott N, Berger S et al (2021) A randomised, placebo-controlled study of RIPK1 inhibitor GSK2982772 in patients with active ulcerative colitis. BMJ Open Gastroenterol 8(1):e000680
Weisel K, Berger S, Thorn K et al (2021) A randomized, placebo-controlled experimental medicine study of RIPK1 inhibitor GSK2982772 in patients with moderate to severe rheumatoid arthritis. Arthritis Res Ther 23(1):85
Harris PA, Marinis JM, Lich JD et al (2019) Identification of a RIP1 kinase inhibitor clinical candidate (GSK3145095) for the treatment of pancreatic cancer. ACS Med Chem Lett 10(6):857–862
Yang X, Lu H, Xie H et al (2022) Potent and selective RIPK1 inhibitors targeting dual-pockets for the treatment of systemic inflammatory response syndrome and sepsis. Angew Chem Int Ed Engl 61(5):e202114922
Yoshikawa M, Saitoh M, Katoh T et al (2018) Discovery of 7-oxo-2,4,5,7-tetrahydro-6 H-pyrazolo[3,4-c]pyridine derivatives as potent, orally available, and brain-penetrating receptor interacting protein 1 (RIP1) kinase inhibitors: analysis of structure-kinetic relationships. J Med Chem 61(6):2384–2409
Berger SB, Harris P, Nagilla R et al (2015) Characterization of GSK’963: a structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov 1:15009
Wang W, Marinis JM, Beal AM et al (2018) RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell 34(5):757-774.e7
Chen L, Zhang X, Ou Y et al (2022) Advances in RIPK1 kinase inhibitors. Front Pharmacol 13:976435
Hou J, Ju J, Zhang Z et al (2019) Discovery of potent necroptosis inhibitors targeting RIPK1 kinase activity for the treatment of inflammatory disorder and cancer metastasis. Cell Death Dis 10(7):493
Ren Y, Su Y, Sun L et al (2017) Discovery of a highly potent, selective, and metabolically stable inhibitor of receptor-interacting protein 1 (RIP1) for the treatment of systemic inflammatory response syndrome. J Med Chem 60(3):972–986
Delehouze C, Leverrier-Penna S, Le Cann F et al (2017) 6E11, a highly selective inhibitor of receptor-interacting protein kinase 1, protects cells against cold hypoxia-reoxygenation injury. Sci Rep 7(1):12931
Delehouze C, Comte A, Leon-Icaza SA et al (2022) Nigratine as dual inhibitor of necroptosis and ferroptosis regulated cell death. Sci Rep 12(1):5118
Patel S, Webster JD, Varfolomeev E et al (2020) RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ 27(1):161–175
Vissers M, Heuberger J, Groeneveld GJ et al (2022) Safety, pharmacokinetics and target engagement of novel RIPK1 inhibitor SAR443060 (DNL747) for neurodegenerative disorders: randomized, placebo-controlled, double-blind phase I/Ib studies in healthy subjects and patients. Clin Transl Sci 15(8):2010–2023
Mandal P, Berger SB, Pillay S et al (2014) RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56(4):481–495
Zhou T, Wang Q, Phan N et al (2019) Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models. Cell Death Dis 10(3):226
Yang XS, Yi TL, Zhang S et al (2017) Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep-Uk. https://doi.org/10.1038/s41598-017-06088-0
Kim SY, Park S, Lee SW et al (2021) RIPK3 contributes to lyso-Gb3-induced podocyte death. Cells 10(2):245
Chen D, Gregory AD, Li X et al (2021) RIP3-dependent necroptosis contributes to the pathogenesis of chronic obstructive pulmonary disease. JCI Insight. https://doi.org/10.1172/jci.insight.144689
Rodriguez DA, Weinlich R, Brown S et al (2016) Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ 23(1):76–88
Xia K, Zhu F, Yang C et al (2020) Discovery of a potent RIPK3 inhibitor for the amelioration of necroptosis-associated inflammatory injury. Front Cell Dev Biol 8:606119
Wu S, Xu C, Xia K et al (2021) Ring closure strategy leads to potent RIPK3 inhibitors. Eur J Med Chem 217:113327
Park HH, Park SY, Mah S et al (2018) HS-1371, a novel kinase inhibitor of RIP3-mediated necroptosis. Exp Mol Med 50(9):1–15
Horvath C, Young M, Jarabicova I et al (2021) Inhibition of cardiac RIP3 mitigates early reperfusion injury and calcium-induced mitochondrial swelling without altering necroptotic signalling. Int J Mol Sci 22(15):7983
Xu CH, Wang JN, Suo XG et al (2022) RIPK3 inhibitor-AZD5423 alleviates acute kidney injury by inhibiting necroptosis and inflammation. Int Immunopharmacol 112:109262
Li JX, Feng JM, Wang Y et al (2014) The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis 5:e1278
Martens S, Jeong M, Tonnus W et al (2017) Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury. Cell Death Dis 8(6):e2904
Chen X, Zhuang C, Ren Y et al (2019) Identification of the Raf kinase inhibitor TAK-632 and its analogues as potent inhibitors of necroptosis by targeting RIPK1 and RIPK3. Br J Pharmacol 176(12):2095–2108
Zhang H, Xu L, Qin X et al (2019) N-(7-Cyano-6-(4-fluoro-3-(2-(3-(trifluoromethyl)phenyl)acetamido)phenoxy)benzo[d] thiazol-2-yl)cyclopropanecarboxamide (TAK-632) analogues as novel necroptosis inhibitors by targeting receptor-interacting protein kinase 3 (RIPK3): synthesis, structure-activity relationships, and in vivo efficacy. J Med Chem 62(14):6665–6681
Hart AC, Abell L, Guo J et al (2020) Identification of RIPK3 type II inhibitors using high-throughput mechanistic studies in hit triage. ACS Med Chem Lett 11(3):266–271
Kim KS, Zhang L, Schmidt R et al (2008) Discovery of pyrrolopyridine-pyridone based inhibitors of Met kinase: synthesis, X-ray crystallographic analysis, and biological activities. J Med Chem 51(17):5330–5341
Schroeder GM, An Y, Cai ZW et al (2009) Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem 52(5):1251–1254
Cui B, Yan B, Wang K et al (2022) Discovery of a new class of uracil derivatives as potential mixed lineage kinase domain-like protein (MLKL) inhibitors. J Med Chem. https://doi.org/10.1021/acs.jmedchem.2c00548
Duan X, Liu X, Liu N et al (2020) Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis 11(2):134
He F, Zheng G, Hu J et al (2022) Necrosulfonamide improves post-resuscitation myocardial dysfunction via inhibiting pyroptosis and necroptosis in a rat model of cardiac arrest. Eur J Pharmacol 926:175037
Yang W, Tao K, Wang Y et al (2022) Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem Pharmacol 206:115338
Zhang X, Zhang Y, Wang F et al (2022) Necrosulfonamide alleviates acute brain injury of intracerebral hemorrhage via inhibiting inflammation and necroptosis. Front Mol Neurosci 15:916249
Rathkey JK, Zhao J, Liu Z et al (2018) Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. https://doi.org/10.1126/sciimmunol.aat2738
Rashidi M, Simpson DS, Hempel A et al (2019) The pyroptotic cell death effector gasdermin D is activated by gout-associated uric acid crystals but is dispensable for cell death and IL-1beta release. J Immunol 203(3):736–748
Sun L, Wang X (2014) A new kind of cell suicide: mechanisms and functions of programmed necrosis. Trends Biochem Sci 39(12):587–593
Yan B, Liu L, Huang S et al (2017) Discovery of a new class of highly potent necroptosis inhibitors targeting the mixed lineage kinase domain-like protein. Chem Commun (Camb) 53(26):3637–3640
Hildebrand JM, Tanzer MC, Lucet IS et al (2014) Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA 111(42):15072–15077
Ma B, Marcotte D, Paramasivam M et al (2016) ATP-competitive MLKL binders have no functional impact on necroptosis. PLoS ONE 11(11):e0165983
Zhong CS, Zeng B, Qiu JH et al (2022) Gout-associated monosodium urate crystal-induced necrosis is independent of NLRP3 activity but can be suppressed by combined inhibitors for multiple signaling pathways. Acta Pharmacol Sin 43(5):1324–1336
Dai X, Ma R, Jiang W et al (2022) Enterococcus faecalis-induced macrophage necroptosis promotes refractory apical periodontitis. Microbiol Spectr 10:e0104522
Pierotti CL, Tanzer MC, Jacobsen AV et al (2020) Potent inhibition of necroptosis by simultaneously targeting multiple effectors of the pathway. ACS Chem Biol 15(10):2702–2713
Prajapati S, Tomar B, Srivastava A et al (2021) 6,7-Dihydroxycoumarin ameliorates crystal-induced necroptosis during crystal nephropathies by inhibiting MLKL phosphorylation. Life Sci 271:119193
Petrie EJ, Birkinshaw RW, Koide A et al (2020) Identification of MLKL membrane translocation as a checkpoint in necroptotic cell death using monobodies. Proc Natl Acad Sci USA 117(15):8468–8475
Jacobsen AV, Silke J (2016) The importance of being chaperoned: HSP90 and necroptosis. Cell Chem Biol 23(2):205–207
Li D, Xu T, Cao Y et al (2015) A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA 112(16):5017–5022
Bigenzahn JW, Fauster A, Rebsamen M et al (2016) An inducible retroviral expression system for tandem affinity purification mass-spectrometry-based proteomics identifies mixed lineage kinase domain-like protein (MLKL) as an heat shock protein 90 (HSP90) client. Mol Cell Proteomics 15(3):1139–1150
Jacobsen AV, Lowes KN, Tanzer MC et al (2016) HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 7:e2051
Patki JM, Pawar SS (2013) HSP90: chaperone-me-not. Pathol Oncol Res 19(4):631–640
Li D, Li C, Li L et al (2016) Natural product kongensin A is a non-canonical HSP90 inhibitor that blocks RIP3-dependent necroptosis. Cell Chem Biol 23(2):257–266
Johnston AN, Ma Y, Liu H et al (2020) Necroptosis-blocking compound NBC1 targets heat shock protein 70 to inhibit MLKL polymerization and necroptosis. Proc Natl Acad Sci USA 117(12):6521–6530
Johnston AN, Wang Z (2020) HSP70 promotes MLKL polymerization and necroptosis. Mol Cell Oncol 7(5):1791561
Acknowledgements
This work was supported by grants from Tongji University Students Innovation Training Program (No. X2022212), the National Natural Science Foundation of China (No. 32170748), the Shanghai Committee of Science and Technology (No. 21490714300), the Key Research and Development Program of Ningxia (No. 2022BFH02012) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
ZC had the idea for the review article. YZ, ZC, QH, JY, YZ and YH performed the literature search. YZ, ZC, QH, JY, YZ and YH drafted the manuscript. ZC critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Cai, Z., Zhai, Y. et al. Necroptosis inhibitors: mechanisms of action and therapeutic potential. Apoptosis 29, 22–44 (2024). https://doi.org/10.1007/s10495-023-01905-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01905-6