Abstract
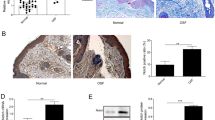
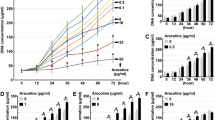
Oral submucous fibrosis (OSF) is an oral condition characterized by chronic progression, which may lead to the development of malignancy. Currently, available treatments for OSF only provide temporary relief of symptoms, and there is a limited availability of effective interventions that can effectively cure this condition. In this study, we aimed to investigate whether adiponectin (APN) could ameliorate OSF and the mechanisms involved in it. First, human oral mucosal fibroblasts (HOMFs) were cultured, an OSF model was established using arecoline, and APN and Imiquimod treatment were administered. Then we overexpressed NLRP3 and knocked down FOXO3A. FOXO3A, fibrosis-related factors (ɑ-SMA, COL1A, CTGF), TGF-β1/Smad3 signaling-related factors (TGF-β1, p-Smad3, Smad3), NLRP3 inflammasome-related factors (NLRP3, Caspase-1, IL-1β), and ROS levels were evaluated. Finally, we explored the effect of APN on OSF in mice by in vivo experiments. We found that arecoline significantly increased ɑ-SMA, COL1A, CTGF, and TGF-β1 expressions and promoted Smad3 phosphorylation, while APN significantly inhibited the elevation of these fibrosis-related factors. ROS production was significantly elevated in HOMFs after arecoline treatment, while APN treatment inhibited ROS production. However, the addition of Imiquimod and overexpression of NLRP3 exhibited a trend of elevated ROS, resisting the inhibitory effect of APN. Furthermore, adding Imiquimod and overexpression of NLRP3 elevated ɑ-SMA, COL1A and CTGF and activated TGF-β1/Smad3 signaling pathway. Additionally, knockdown of FOXO3A enhanced APN-inhibited ɑ-SMA and COL1A. In vivo experiments further confirmed that APN ameliorated OSF in mice by inhibiting ROS/NLRP3 inflammatory pathway. In conclusion, APN ameliorated arecoline-induced OSF by promoting FOXO3A expression and downregulating the ROS/NLRP3 pathway.








Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Qin X, Ning Y, Zhou L, Zhu Y. Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24054992.
Shih YH, Wang TH, Shieh TM, Tseng YH. Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20122940.
Shen YW, Shih YH, Fuh LJ, Shieh TM. Oral submucous fibrosis: a review on biomarkers, pathogenic mechanisms, and treatments. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21197231.
Peng Q, Li H, Chen J, Wang Y, Tang Z. Oral submucous fibrosis in Asian countries. J Oral Pathol Med. 2020;49:294–304.
Tilakaratne WM, Ekanayaka RP, Warnakulasuriya S. Oral submucous fibrosis: a historical perspective and a review on etiology and pathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:178–91.
Chou MY, Hsieh PL, Chao SC, Liao YW, Yu CC, Tsai CY. MiR-424/TGIF2-Mediated Pro-Fibrogenic Responses in Oral Submucous Fibrosis. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24065811.
Liu B, Shen M, Xiong J, Yuan Y, Wu X, Gao X, et al. Synergistic effects of betel quid chewing, tobacco use (in the form of cigarette smoking), and alcohol consumption on the risk of malignant transformation of oral submucous fibrosis (OSF): a case-control study in Hunan Province, China. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:337–45.
Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW. Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol Head Neck Surg. 2020;49:3.
Xu X, Huang X, Zhang L, Huang X, Qin Z, Hua F. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-kappaB/NLRP3 inflammation pathway. BMC Nephrol. 2021;22:218.
Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8:1031–63.
Qi GM, Jia LX, Li YL, Li HH, Du J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155:2254–65.
Wang X, Yang J, Wu L, Tong C, Zhu Y, Cai W, et al. Adiponectin inhibits the activation of lung fibroblasts and pulmonary fibrosis by regulating the nuclear factor kappa B (NF-κB) pathway. Bioengineered. 2022;13:10098–110.
Xie M, Xiong Z, Yin S, Xiong J, Li X, Jin L, et al. Adiponectin alleviates intestinal fibrosis by enhancing AMP-activated protein kinase phosphorylation. Dig Dis Sci. 2022;67:2232–43.
Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18:1141–60.
Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114–27.
Artlett CM. The mechanism and regulation of the NLRP3 inflammasome during fibrosis. Biomolecules. 2022. https://doi.org/10.3390/biom12050634.
Coll RC, Holley CL, Schroder K. Mitochondrial DNA synthesis fuels NLRP3 activation. Cell Res. 2018;28:1046–7.
Luo T, Zhou X, Qin M, Lin Y, Lin J, Chen G, et al. Corilagin restrains NLRP3 inflammasome activation and pyroptosis through the ROS/TXNIP/NLRP3 pathway to prevent inflammation. Oxid Med Cell Longev. 2022;2022:1652244.
Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–5.
Lee SS, Chen YJ, Tsai CH, Huang FM, Chang YC. Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation. Clin Oral Investig. 2016;20:1029–34.
Tan WQ, Wang K, Lv DY, Li PF. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem. 2008;283:29730–9.
Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13:815–27.
Kim J, Toda T, Watanabe K, Shibuya S, Ozawa Y, Izuo N, et al. Syringaresinol reverses age-related skin atrophy by suppressing FoxO3a-mediated matrix metalloproteinase-2 activation in copper/zinc superoxide dismutase-deficient mice. J Invest Dermatol. 2019;139:648–55.
Bošković M, Živković M, Korićanac G, Stanišić J, Zec M, Krga I, et al. Walnut supplementation restores the SIRT1-FoxO3a-MnSOD/Catalase axis in the heart, promotes an anti-inflammatory fatty acid profile in plasma, and lowers blood pressure on fructose-rich diet. Oxid Med Cell Longev. 2021;2021:5543025.
Rajamanickam V, Yan T, Wu L, Zhao Y, Xu X, Zhu H, et al. Allylated curcumin analog CA6 inhibits TrxR1 and leads to ROS-dependent apoptotic cell death in gastric cancer through Akt-FoxO3a. Cancer Manag Res. 2020;12:247–63.
Shrestha A, Park PH. Globular adiponectin attenuates LPS-induced reactive oxygen species production in HepG2 cells via FoxO3A and HO-1 signaling. Life Sci. 2016;148:71–9.
Zeng Y, Liang H, Guo Y, Feng Y, Yao Q. Adiponectin regulates osteocytic MLO-Y4 cell apoptosis in a high-glucose environment through the AMPK/FoxO3a signaling pathway. J Cell Physiol. 2021;236:7088–96.
Xu X, Huang X, Zhang L, Huang X, Qin Z, Hua F. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-κB/NLRP3 inflammation pathway. BMC Nephrol. 2021;22:218.
Chiang MH, Lee KT, Chen CH, Chen KK, Wang YH. Photobiomodulation therapy inhibits oral submucous fibrosis in mice. Oral Dis. 2020;26:1474–82.
Groß CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, et al. K(+) Efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45:761–73.
Zhang L, Tan J, Liu YP, Liu X, Luo M. Curcumin relieves the arecoline-induced fibrosis of oral mucosal fibroblasts via inhibiting HIF-1alpha/TGF-beta/CTGF signaling pathway: an in vitro study. Toxicol Res (Camb). 2021;10:631–8.
Chattopadhyay A, Ray JG. Molecular pathology of malignant transformation of oral submucous fibrosis. J Environ Pathol Toxicol Oncol. 2016;35:193–205.
Xu H, Lyu FY, Song JY, Xu YM, Jiang EH, Shang ZJ, et al. Research achievements of oral submucous fibrosis: progress and prospect. Biomed Res Int. 2021;2021:6631856.
Kuo MY, Chen HM, Hahn LJ, Hsieh CC, Chiang CP. Collagen biosynthesis in human oral submucous fibrosis fibroblast cultures. J Dent Res. 1995;74:1783–8.
Keen AN, Fenna AJ, McConnell JC, Sherratt MJ, Gardner P, Shiels HA. The dynamic nature of hypertrophic and fibrotic remodeling of the fish ventricle. Front Physiol. 2015;6:427.
Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–44.
Deng YT, Chen HM, Cheng SJ, Chiang CP, Kuo MY. Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: Modulation by curcumin. Oral Oncol. 2009;45:e99–105.
Kavitha L, Ranganathan K, Shyam S, Fathima JHS, Umesh W, Warnakulasuriya S. Immunohistochemical biomarkers in oral submucous fibrosis: A scoping review. J Oral Pathol Med. 2022;51:594–602.
Wang W, Xiong H, Hu Z, Zhao R, Hu Y, Chen W, et al. Experimental study on TGF-β1-mediated CD147 expression in oral submucous fibrosis. Oral Dis. 2018;24:993–1000.
Jing H, Tang S, Lin S, Liao M, Chen H, Fan Y, et al. Adiponectin in renal fibrosis. Aging (Albany NY). 2020;12:4660–72.
Yao R, Cao Y, He YR, Lau WB, Zeng Z, Liang ZA. Adiponectin attenuates lung fibroblasts activation and pulmonary fibrosis induced by paraquat. PLoS ONE. 2015;10:e0125169.
Zhao D, Zhu X, Jiang L, Huang X, Zhang Y, Wei X, et al. Advances in understanding the role of adiponectin in renal fibrosis. Nephrology (Carlton). 2021;26:197–203.
Das A, Giri S. A review on role of arecoline and its metabolites in the molecular pathogenesis of oral lesions with an insight into current status of its metabolomics. Prague Med Rep. 2020;121:209–35.
Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–9.
He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–21.
Dong Z, Zhuang Q, Ye X, Ning M, Wu S, Lu L, et al. Adiponectin inhibits NLRP3 inflammasome activation in nonalcoholic steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS signaling pathways. Front Med (Lausanne). 2020;7:546445.
Wang F, Liu Y, Yang W, Yuan J, Mo Z. Adiponectin inhibits NLRP3 inflammasome by modulating the AMPK-ROS pathway. Int J Clin Exp Pathol. 2018;11:3338–47.
Funding
This work was supported by the Clinical Medical Technology Innovation Guidance Project (No. 2021SK52004).
Author information
Authors and Affiliations
Contributions
YZ and ML contributed to conceptualization, data curation, formal analysis, validation, writing of the original draft. ZY contributed to investigation, software and methodology. XX contributed to funding acquisition, project administration, supervision and review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
Ethics approval
This study was approved by Hunan SJA Laboratory Animal Co., Ltd. (No. SJA202302001: the license number) and conducted in strict accordance with the national institutes of health guidelines for the care and use of experimental animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Y., Luo, M., Yao, Z. et al. Adiponectin inhibits ROS/NLRP3 inflammatory pathway through FOXO3A to ameliorate oral submucosal fibrosis. Odontology (2024). https://doi.org/10.1007/s10266-023-00891-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10266-023-00891-0