Abstract
Background
Neoadjuvant docetaxel, cisplatin, and 5-fluorouracil (DCF) therapy is a new standard for locally advanced esophageal squamous cell carcinoma. The optimal timing of pegfilgrastim with the DCF regimen to prevent febrile neutropenia (FN) remains controversial. The effectiveness of concomitant pegfilgrastim administration with continuous 5-fluorouracil (5-FU) infusion in the DCF regimen was therefore assessed.
Methods
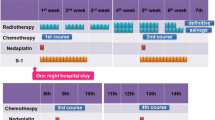
All patients who received neoadjuvant DCF for esophageal cancer were retrospectively assessed. Patients who had been scheduled to receive pegfilgrastim on days 3–5 (early group) or days 7–9 (regular group) of the DCF regimen were included. Uni- and multivariate analyses were used to assess risk factors for FN.
Results
Eighty-eight patients were included in the analysis. The 26 patients in the early group received pegfilgrastim as scheduled. In the 62 patients of the regular group, 51 received pegfilgrastim at a median of 7 days after starting DCF chemotherapy. However, 11 patients in the regular group could not receive pegfilgrastim. Twenty-two patients of the regular group and 2 patients of the early group developed FN after the first session of DCF. Early administration of pegfilgrastim and grade 4 neutropenia were significantly associated with onset of FN, with multivariate analysis identifying early administration of pegfilgrastim as an independent preventive factor and grade 4 neutropenia as a risk factor, after adjusting for sex and age.
Conclusion
Early pegfilgrastim administration is a safe approach that reduces the incidence of FN in DCF therapy. Using pegfilgrastim with continuous 5-FU infusion in the DCF regimen represents a reasonable option to prevent FN.
Similar content being viewed by others
References
Uhlenhopp DJ, Then EO, Sunkara T et al (2020) Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol 13:1010–1021
Ilson DH, van Hillegersberg R (2018) Management of patients with adenocarcinoma or squamous cancer of the esophagus. Gastroenterology 154:437–451
Eyck BM, van Lanschot JJB, Hulshof M et al (2021) Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled cross trial. J Clin Oncol 39:1995–2004
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084
Matsuda S, Kitagawa Y, Takemura R et al (2022) Real-world evaluation of the efficacy of neoadjuvant dcf over cf in esophageal squamous cell carcinoma: propensity score matched analysis from 85 authorized institutes for esophageal cancer in japan. Ann Surg 278:e35
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Yokota T, Kato K, Hamamoto Y et al (2016) Phase ii study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer 115:1328–1334
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of wbc growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
Curran MP, Goa KL (2002) Pegfilgrastim. Drugs 62:1207–1213 (discussion 1214–1205)
Maeda O, Fukaya M, Koike M et al (2022) Preoperative docetaxel, cisplatin, and fluorouracil treatment with pegfilgrastim on day 7 for patients with esophageal cancer: a phase ii study. Asia Pac J Clin Oncol 18:578–585
Ishikawa T, Yasuda T, Okayama T et al (2019) Early administration of pegfilgrastim for esophageal cancer treated with docetaxel, cisplatin, and fluorouracil: a phase ii study. Cancer Sci 110:3754–3760
Schnipper LE, Davidson NE, Wollins DS et al (2015) American society of clinical oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 33:2563–2577
Obermannova R, Alsina M, Cervantes A et al (2022) Oesophageal cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33:992–1004
Klastersky J (2004) Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis 39(Suppl 1):S32-37
Yamasaki M, Miyata H, Tanaka K et al (2011) Multicenter phase i/ii study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology 80:307–313
Takahashi H, Arimura Y, Yamashita K et al (2010) Phase i/ii study of docetaxel/cisplatin/fluorouracil combination chemotherapy against metastatic esophageal squamous cell carcinoma. J Thorac Oncol 5:122–128
Osaka Y, Shinohara M, Hoshino S et al (2011) Phase ii study of combined chemotherapy with docetaxel, cddp and 5-fu for highly advanced esophageal cancer. Anticancer Res 31:633–638
Noronha V, Joshi A, Jandyal S et al (2014) High pathologic complete remission rate from induction docetaxel, platinum and fluorouracil (dcf) combination chemotherapy for locally advanced esophageal and junctional cancer. Med Oncol 31:188
Patel K, West HJ (2017) Febrile neutropenia. JAMA Oncol 3:1751
Kuderer NM, Dale DC, Crawford J et al (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Lyman GH (2005) Guidelines of the national comprehensive cancer network on the use of myeloid growth factors with cancer chemotherapy: a review of the evidence. J Natl Compr Canc Netw 3:557–571
Lyman GH, Dale DC, Culakova E et al (2013) The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 24:2475–2484
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of eortc guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Holmes FA, O’Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage ii or stage iii/iv breast cancer. J Clin Oncol 20:727–731
Kawahira M, Yokota T, Hamauchi S et al (2018) Primary prophylactic granulocyte colony-stimulating factor according to asco guidelines has no preventive effect on febrile neutropenia in patients treated with docetaxel, cisplatin, and 5-fluorouracil chemotherapy. Int J Clin Oncol 23:1189–1195
Yoshida Y, Komori K, Aoki M et al (2018) Efficacy of pegfilgrastim administration in patients with esophageal cancer treated with docetaxel, cisplatin, and 5-fluorouracil. Pharmazie 73:613–616
Ma X, Kang J, Li Y et al (2021) Pegfilgrastim safety and efficacy on the last chemotherapy day versus the next: systematic review and meta-analysis. BMJ Support Palliat Care:bmjspcare-2020-002532
Linot B, Augereau P, Breheret R et al (2014) Efficacy and safety of early g-csf administration in patients with head and neck cancer treated by docetaxel-cisplatin and 5-fluorouracil (dcf protocol): a retrospective study. Support Care Cancer 22:2831–2837
Endo Y, Ishikawa T, Oka K et al (2022) Effect of concomitant use of g-csf and myelosuppressive chemotherapy on bone marrow and peripheral granulocytes in a mouse model. Med Oncol 39:110
Janoray G, Pointreau Y, Garaud P et al (2016) Long-term results of a multicenter randomized phase iii trial of induction chemotherapy with cisplatin, 5-fluorouracil, +/- docetaxel for larynx preservation. J Natl Cancer Inst 108:djv368. https://doi.org/10.1093/jnci/djv368
Posner MR, Norris CM, Wirth LJ et al (2009) Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in tax 324: Survival, surgery, and organ preservation. Ann Oncol 20:921–927
Cho H, Nishiike S, Yamamoto Y et al (2015) Docetaxel, cisplatin, and fluorouracil for patients with inoperable recurrent or metastatic head and neck squamous cell carcinoma. Auris Nasus Larynx 42:396–400
Takenaka Y, Cho H, Yamamoto M et al (2013) Incidence and predictors of febrile neutropenia during chemotherapy in patients with head and neck cancer. Support Care Cancer 21:2861–2868
Schuman SI, Lambrou N, Robson K et al (2009) Pegfilgrastim dosing on same day as myelosuppressive chemotherapy for ovarian or primary peritoneal cancer. J Support Oncol 7:225–228
Whitworth JM, Matthews KS, Shipman KA et al (2009) The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. Gynecol Oncol 112:601–604
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TY and TO. The first draft of the manuscript was written by TY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshimoto, T., Oshima, T., Fukada, T. et al. Pegfilgrastim for the management of neutropenia during neoadjuvant chemotherapy with docetaxel, cisplatin, and 5-fluorouracil in esophageal cancer patients. Int J Clin Oncol 29, 142–148 (2024). https://doi.org/10.1007/s10147-023-02438-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02438-3