Abstract
Background
S-1 plus cisplatin (SP) and capecitabine plus cisplatin (XP) are standard first-line regimens for advanced gastric cancer (AGC) worldwide. We conducted a meta-analysis using individual participant data (IPD) to investigate which is more suitable.
Methods
IPD from three randomized trials were collected. In these trials, patients with AGC were randomly allocated to SP (S-1 80–120 mg for 21 days plus cisplatin 60 mg/m2 (q5w)) or XP (capecitabine 2000 mg/m2 for 14 days plus cisplatin 80 mg/m2 (q3w)).
Results
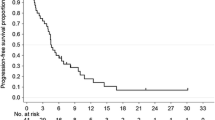
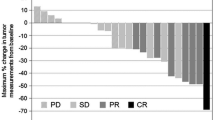
In 211 eligible patients, median overall survival (OS) for SP versus XP was 13.5 and 11.7 months (hazard ratio [HR], 0.787; p = 0.114), progression-free survival (PFS) was 6.2 and 5.1 months (HR, 0.767; P = 0.076), and TTF was 5.1 and 4.0 months (HR, 0.611; P = 0.001). The most common grade ≥ 3 adverse events with SP or XP were neutropenia (18% vs. 29%) and anorexia (16% vs.18%). Subgroup analysis demonstrated significant interaction between treatment effect and performance status > 1 (HR, 0.685; P = 0.036), measurable lesion (HR, 0.709; P = 0.049), primary upper third tumor (HR, 0.539; P = 0.040), and differentiated type (HR, 0.549; interaction, 0.236; P = 0.019). For the differentiated type, OS was significantly longer in the SP group (13.2 months) than in the XP group (11.1 months) (HR, 0.549; P = 0.019). For the undifferentiated type, OS was similar in the SP group (14.2 months) and in the XP group (12.4 months) (HR, 0.868; P = 0.476).
Conclusions
SP and XP were both effective and well tolerated. SP might be suitable for the pathological differentiated subtype of AGC.
Clinical Trial Registration: The HERBIS-2, HERBIS-4A, and XParTS II trials were registered with UMIN-CTR as UMIN000006105, UMIN000006755, and UMIN000006045, respectively.




Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Wagner AD, Grothe W, Haerting J et al (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol 24:4991–4997
Cunnigham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Ohtsu A, Shah MA, Van Cutsem E et al (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29:3968–3976
Lordick F, Kang YK, Chung HC et al (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14:490–499
Japanese gastric cancer treatment guidelines (2018) 5th edition. Gastric Cancer 2021(24):1–21
Nishikawa K, Tsuburaya A, Yoshikawa T et al (2018) A randomised phase II trial of capecitabine plus cisplatin versus S-1 plus cisplatin as a first-line treatment for advanced gastric cancer: Capecitabine plus cisplatin ascertainment versus S-1 plus cisplatin randomised PII trial (XParTS II). Eur J Cancer 101:220–228
Kawakami H, Fujitani K, Matsuyama J et al (2020) Comparison of S-1-cisplatin every 5 weeks with capecitabine-cisplatin every 3 weeks for HER2-negative gastric cancer (recurrent after S-1 adjuvant therapy or chemotherapy-naive advanced): pooled analysis of HERBIS-2 (OGSG 1103) and HERBIS-4A (OGSG 1105) trials. Int J Clin Oncol 25:1635–1643
Kawakami H, Takeno A, Endo S et al (2018) Randomized, open-label phase ii study comparing capecitabine-cisplatin every 3 weeks with S-1-cisplatin every 5 weeks in chemotherapy-naive patients with HER2-negative advanced gastric cancer: OGSG1105, HERBIS-4A trial. Oncologist 23:1411-e147
Lee JL, Kang YK, Kang HJ et al (2008) A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 99:584–90
Kim MJ, Kong SY, Nam BH et al (2018) A randomized phase II study of S-1 versus capecitabine as first-line chemotherapy in elderly metastatic gastric cancer patients with or without poor performance status: clinical and pharmacogenetic results. Pharmacogenet Genom 28:23–30
Kim GM, Jeung HC, Rha SY et al (2012) A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 48:518–526
He MM, Wu WJ, Wang F et al (2013) S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for advanced gastric carcinoma: a meta-analysis. PLoS ONE 8:e82798
Ter Veer E, Mohammad NH, Lodder P et al (2016) The efficacy and safety of S-1-based regimens in the first-line treatment of advanced gastric cancer: a systematic review and meta-analysis. Gastric Cancer 19:696–712
Feng Z, Yan P, Hou X et al (2020) The efficacy and safety of capecitabine-based versus S-1-based chemotherapy for metastatic or recurrent gastric cancer: a systematic review and meta-analysis of clinical randomized trials. Ann Palliat Med 9:883–894
Stewart LA, Clarke M, Rovers M et al (2015) Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 313:1657–65
Kawakami H, Nishikawa K, Shimokawa T et al (2022) Histology classification highlights differences in efficacy of S-1 versus capecitabine, in combination with cisplatin, for her2-negative unresectable advanced or recurrent gastric cancer with measurable disease. Cancers 14:5673
Ryu MH, Baba E, Lee KH et al (2015) Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol 26:2097–2101
Takashima A, Iizumi S, Boku N (2017) Survival after failure of first-line chemotherapy in advanced gastric cancer patients: differences between Japan and the rest of the world. Jpn J Clin Oncol 47:583–589
Cancer Genome Atlas Research N (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517):202–209
Ajani JA, Rodriguez W, Bodoky G et al (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28:1547–1553
Ajani JA, Abramov M, Bondarenko I et al (2017) A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol 28:2142–2148
Shirasaka T, Shimamato Y, Ohshimo H et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–57
Yamada Y, Higuchi K, Nishikawa K et al (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 26:141–148
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet 398:27–40
Kang YK, Chen LT, Ryu MH et al (2022) Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23:234–247
Boku N, Ryu MH, Kato K et al (2019) Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 30:250–58
Acknowledgements
We deeply appreciate all patients and investigators involved in the XParTS II trial and the OGSG HEBIS-2, -4A trials. We also thank the Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG) and the Epidemiological & Clinical Research Information Network (ECRIN) for their support.
Funding
This study was funded by the Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG). [No grant numbers apply].
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interests
Kazuhiro Nishikawa has received honoraria for lectures from Bristol-Myers Squibb Co. Ltd., Daiichi-Sankyo Co. Ltd., EA Pharma Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. Hisato Kawakami has received consulting fees from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., and Daiichi-Sankyo Co. Ltd.; honoraria from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., and Daiichi Sankyo Co. Ltd. Yukinori Kurokawa has received honoraria and research funding from Taiho Pharmaceutical. Taroh Satoh has received departmental research grants from Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Daiichi-Sankyo Co. Ltd., Hutchmed, Parexell, and BeiGene, and honoraria from Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., and Daiichi-Sankyo Co. Ltd. All remaining authors declare no conflicts of interest.
Ethical approval
This trial was conducted in compliance with the ethical principles of the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of the Japanese Ministry of Health, Labour and Welfare. This trial was approved by the institutional review boards or ethics committees at all participating centers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2023_2402_MOESM1_ESM.docx
Supplementary file 1 Fig. S1. Kaplan–Meier curves for PFS in differentiated type (a) and in undifferentiated type (b) by treatment arm. PFS, progression-free survival; SP, S-1 plus cisplatin; XP, capecitabine plus cisplatin.
About this article
Cite this article
Nishikawa, K., Kawakami, H., Shimokawa, T. et al. Meta-analysis of three randomized trials of capecitabine plus cisplatin (XP) versus S-1 plus cisplatin (SP) as first-line treatment for advanced gastric cancer. Int J Clin Oncol 28, 1501–1510 (2023). https://doi.org/10.1007/s10147-023-02402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02402-1