Abstract
Background
We previously reported the HERBIS-4A phase II trial comparing S-1 plus cisplatin (SP) with capecitabine plus cisplatin (XP) in chemotherapy-naïve patients with HER2-negative advanced gastric cancer (GC). We performed a pooled analysis of HERBIS-4A and HERBIS-2, the phase II trial comparing SP with XP in HER2-negative recurrent GC patients with a recurrence-free interval after S-1 adjuvant therapy of ≥ 6 months.
Patients and methods
Patients were randomly assigned to receive either SP [S-1 (40–60 mg twice daily for 21 days) plus cisplatin (60 mg/m2 on day 8), every 5 weeks] or XP [capecitabine (1000 mg/m2 twice daily for 14 days) plus cisplatin (80 mg/m2 on day 1), every 3 weeks].
Results
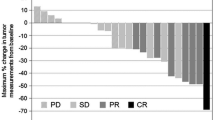
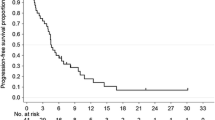
In the pooled analysis, SP (n = 44–50) showed a longer progression-free survival [6.4 versus 5.1 months; hazard ratio (HR), 0.666; P = 0.062], overall survival (14.8 versus 10.6 months; HR, 0.695; P = 0.099), and time to treatment failure (4.6 versus 3.6 months; HR, 0.668; P = 0.045) as well as a higher disease control rate (86.4% versus 68.1%, P = 0.149) compared with XP (n = 47–51). A significant survival advantage for SP over XP was apparent in patients with a performance status of 0, a differentiated-type tumor histology, or a primary tumor localization to the upper portion of the stomach.
Conclusion
Our pooled analysis supports the use of SP in the first-line setting for patients with HER2-negative advanced or recurrent GC with a recurrence-free interval of ≥ 6 months.
Clinical trial registration
The HERBIS-2 trial was registered with UMIN-CTR as UMIN000006105.




Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer J Int Cancer 136(5):E359–386. https://doi.org/10.1002/ijc.29210
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Boku N, Yamamoto S, Fukuda H et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10(11):1063–1069. https://doi.org/10.1016/S1470-2045(09)70259-1
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. https://doi.org/10.1016/S1470-2045(08)70035-4
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial Annals of oncology. Off J Eur Soc Med Oncol ESMO 20(4):666–673. https://doi.org/10.1093/annonc/mdn717
Lordick F, Kang YK, Chung HC et al (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14(6):490–499. https://doi.org/10.1016/S1470-2045(13)70102-5
Ohtsu A, Shah MA, Van Cutsem E et al (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29(30):3968–3976. https://doi.org/10.1200/JCO.2011.36.2236
Kawakami H, Takeno A, Endo S et al (2018) Randomized, open-label phase ii study comparing capecitabine-cisplatin every 3 weeks with s-1-cisplatin every 5 weeks in chemotherapy-naive patients with her2-negative advanced gastric cancer: ogsg1105, herbis-4a trial. Oncologist 23(12):1411–e1147. https://doi.org/10.1634/theoncologist.2018-0175
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820. https://doi.org/10.1056/NEJMoa072252
Shitara K, Morita S, Fujitani K et al (2012) Combination chemotherapy with S-1 plus cisplatin for gastric cancer that recurs after adjuvant chemotherapy with S-1: multi-institutional retrospective analysis. Gastric Cancer 15(3):245–251. https://doi.org/10.1007/s10120-011-0101-x
Nishikawa K, Tsuburaya A, Yoshikawa T et al (2018) A phase II trial of capecitabine plus cisplatin (XP) for patients with advanced gastric cancer with early relapse after S-1 adjuvant therapy: XParTS-I trial. Gastric Cancer 21(5):811–818. https://doi.org/10.1007/s10120-018-0815-0
Sano T, Aiko T (2011) New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points Gastric cancer. Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc 14(2):97–100. https://doi.org/10.1007/s10120-011-0040-6
Cancer Genome Atlas Research N (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517):202–209. https://doi.org/10.1038/nature13480
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Japanese Gastric Cancer A (1998) Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 1 (1):10–24. doi:10.1007/s101209800016
Yamada Y, Boku N, Nishina T et al (2013) Impact of excision repair cross-complementing gene 1 (ERCC1) on the outcomes of patients with advanced gastric cancer: correlative study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol 24(10):2560–2565. https://doi.org/10.1093/annonc/mdt238
Ajani JA, Rodriguez W, Bodoky G et al (2009) Multicenter phase III comparison of cisplatin/S-1 (CS) with cisplatin/5-FU (CF) as first-line therapy in patients with advanced gastric cancer (FLAGS): Secondary and subset analyses. J Clin Oncol 27:4511. https://doi.org/10.1200/jco.2009.27.15_suppl.4511(no. 15_suppl (May 2009)
Ajani JA, Rodriguez W, Bodoky G et al (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28(9):1547–1553. https://doi.org/10.1200/JCO.2009.25.4706
Ajani JA, Abramov M, Bondarenko I et al (2017) A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol Off J Eur Soc Med Oncol ESMO 28(9):2142–2148. https://doi.org/10.1093/annonc/mdx275
Xu R-h, Wang Z-Q, Shen L et al (2019) S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: a randomized, phase 3 trial. J Clin Oncol 37:4017. https://doi.org/10.1200/JCO.2019.37.15_suppl.4017(15_suppl)
Nishikawa K, Tsuburaya A, Yoshikawa T et al (2018) A randomised phase II trial of capecitabine plus cisplatin versus S-1 plus cisplatin as a first-line treatment for advanced gastric cancer: Capecitabine plus cisplatin ascertainment versus S-1 plus cisplatin randomised PII trial (XParTS II). Eur J Cancer 101:220–228. https://doi.org/10.1016/j.ejca.2018.06.026
Acknowledgements
We thank all the patients, investigators, and medical staff at the participating institutions who contributed to this study.
Funding
This research was funded by Osaka Clinical Study Supporting Organization.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
H.K. has received consulting fees from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., and Taiho Pharmaceutical Co. Ltd; honoraria from Bristol-Myers Squibb Co. Ltd., AstraZeneca K.K., Bayer yakuhin Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd.; lecture fees from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co., Ltd.; and research funding from Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd, and Eisai Co. Ltd. T. Tamura has received honoraria from Merck Serono Co. Ltd., speaker’s bureau fees from Daiichi Sankyo Co. Ltd., and research funding from Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. Y. Kurokawa has received lecture fees from Taiho Pharmaceutical Co. Ltd., Yakult Honsha, Ono Pharmaceutical Co. Ltd., MSD K.K., Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Kaken Pharmaceutical, as well as research grants from Taiho Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., and MSD K.K. T. Satoh received research grants from Giliad; consulting fees from Daiichi Sankyo and and Takeda Pharmaceutical, Co. Ltd.; consulting fees, honoraria and research grants from Merck BioPharm, Bristol-Myers K.K., Taiho pharmaceutical, Elli lilly, MSD,, Sanofi, Bristol Myers-Squib; and departmental research grants, research grants, honoraria and consulting fees from Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical. Co. Ltd. and Yakult Honsha Co. Ltd. All remaining authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kawakami, H., Fujitani, K., Matsuyama, J. et al. Comparison of S-1–cisplatin every 5 weeks with capecitabine-cisplatin every 3 weeks for HER2-negative gastric cancer (recurrent after S-1 adjuvant therapy or chemotherapy-naïve advanced): pooled analysis of HERBIS-2 (OGSG 1103) and HERBIS-4A (OGSG 1105) trials. Int J Clin Oncol 25, 1635–1643 (2020). https://doi.org/10.1007/s10147-020-01711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01711-z