Abstract
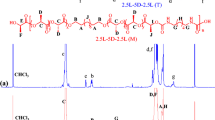
In this work, hydroxyl-terminated oxalamide compounds N1,N2-bis(2-hydroxyethyl)oxalamide (OXA1) and N1, N1′-(ethane-1,2-diyl)bis(N2-(2-hydroxyethyl)oxalamide (OXA2) were synthesized to initiate the ring-opening polymerization of L-lactide for preparation of oxalamide-hybridized poly(L-lactide) (PLAOXA), i.e., PLAOXA1 and PLAOXA2. The crystallization properties of PLA were improved by the self-assembly of the oxalamide segments in PLAOXA which served as the initial heterogeneous nuclei. The crystal growth kinetics was studied by Hoffman-Lauritzen theory and it revealed that the nucleation energy barrier of PLAOXA1 and PLAOXA2 was lower than that of PLA. Consequently, PLAOXA could crystallize much faster than PLA, accompanied with a decrease in spherulite size and half-life crystallization time by 74.8% and 86.5% (T = 125 °C), respectively. In addition, the final crystallinity of PLAOXA1 and PLAOXA2 was 6 and 8 times higher, respectively, in comparison with that of neat PLA under a controlled cooling rate of 10 °C/min. The results demonstrate that the hybridization of oxalamide segments in PLA backbone will serve as the self-heteronucleation for promoting the crystallization rate. The higher the content of oxalamide segments (PLAOXA2 compared with PLAOXA1) is, the stronger the promotion effect will be. Therefore, this study may provide a universal approach by hybridizing macromolecular structure to facilitate the crystallization of semi-crystalline polymer materials.
Similar content being viewed by others
References
Rankin, J. European parliament votes to ban single-use plastics. The Guardian 2019, 27.
Trache, D.; Hussin, M. H.; Haafiz, M. K. M.; Thakur, V. K. Recent progress in cellulose nanocrystals: sources and production. Nanoscale 2017, 9, 1763–1786.
Gu, J.; Peng, X.; Peng, C.; Lei, Z.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Tao, C. Functionalization of biodegradable PLA non-woven fabric as superoleophilic and superhydrophobic material for efficient oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 5968.
Tenn, N.; Follain, N.; Soulestin, J.; Crétois, R.; Bourbigot, S.; Marais, S. Effect of nanoclay hydration on barrier properties of PLA/montmorillonite based nanocomposites. J. Phys. Chem. C 2013, 117, 12117–12135.
Saiwaew, R.; Suppakul, P.; Boonsupthip, W.; Pechyen, C.; Saiwaew, R. Development and characterization of poly(lactic acid)/fish water soluble protein composite sheets: a potential approach for biodegradable packaging. Energy Procedia 2014, 56, 280–298.
Chen, Y.; Wang, W.; Qiu, Y.; Li, L.; Qian, L.; Xin, F. Terminal group effects of phosphazene-triazine bi-group flame retardant additives in flame retardant polylactic acid composites. Polym. Degrad. Stab. 2017, 140, 166–175.
Zhang, X.; Meng, L.; Li, G.; Liang, N.; Zhang, J.; Zhu, Z.; Wang, R. Effect of nucleating agents on the crystallization behavior and heat resistance of poly(l-lactide). J. Appl. Polym. Sci. 2016, 133, 42999.
Zhu, B.; Wang, Y.; Liu, H.; Ying, J.; Liu, C.; Shen, C. Effects of interface interaction and microphase dispersion on the mechanical properties of PCL/PLA/MMT nanocomposites visualized by nanomechanical mapping. Compos. Sci. Technol. 2020, 190, 108048.
Chen, P.; Lian, H.; Shih, Y.; Chenwei, S.; Jeng, R. Preparation, characterization and crystallization kinetics of Kenaf fiber/multi-walled carbon nanotube/polylactic acid (PLA) green composites. Mater. Chem. Phys. 2017, 196, 249–255.
Xu, P.; Cui, Z.; Ruan, G.; Ding, Y. Enhanced crystallization kinetics of PLLA by ethoxycarbonyl ionic liquid modified graphene. Chinese J. Polym. Sci. 2019, 37, 243–252.
Bhoje Gowd, E.; Nagendra, B.; Sivaprasad, V. P.; Sijla Rosely, C. Influence of boron nitride nanosheets on the crystallization and polymorphism of poly(L-lactide). J. Phys. Chem. B 2018, 122, 6442–6451.
Nam, J. Y.; Okamoto, M.; Okamoto, H.; Nakano, M.; Usuki, A.; Matsuda, M. Morphology and crystallization kinetics in a mixture of low-molecular weight aliphatic amide and polylactide. Polymer 2006, 47, 1340–1347.
Pan, P.; Shan, G.; Bao, Y. Enhanced nucleation and crystallization of poly(L-lactic acid) by immiscible blending with poly(vinylidene fluoride). Ind. Eng. Chem. Res. 2014, 53, 3148–3156.
Yuan, B. C.; Zou, L. G. Effect of orotic acid on the crystallization kinetics and morphology of biodegradable poly(L-lactide) as an efficient nucleating agent. Thermochim. Acta 2014, 577, 41–45.
Pan, P.; Yang, J.; Shan, G.; Bao, Y.; Weng, Z.; Inoue, Y. Nucleation effects of nucleobases on the crystallization kinetics of poly(L-lactide). Macromol. Mater. Eng. 2012, 297, 670–679.
Shi, Y.; Shao, L.; Yang, J.; Huang, T.; Wang, Y.; Zhang, N.; Wang, Y. Highly improved crystallization behavior of poly(L-lactide) induced by a novel nucleating agent: substituted-aryl phosphate salts. Polym. Adv. Technol. 2013, 24, 42–50.
Xie, Q.; Han, L.; Shan, G.; Bao, Y.; Pan, P. Polymorphic crystalline structure and crystal morphology of eenantiomeric poly(lactic acid) blends tailored by a self-assemblable aryl amide nucleator. ACS Sustain. Chem. Eng. 2016, 4, 2680–2688.
Ma, P.; Xu, Y.; Wang, D.; Dong, W.; Chen, M. Rapid crystallization of poly(lactic acid) by using tailor-made oxalamide derivatives as novel soluble-type nucleating agents. Ind. Eng. Chem. Res. 2014, 53, 12888–12892.
Ma, P.; Xu, Y.; Shen, T.; Dong, W.; Chen, M.; Lemstra, P. J. Tailoring the crystallization behavior of poly(L-lactide) with self-assembly-type oxalamide compounds as nucleators: 1. Effect of terminal configuration of the nucleators. Eur. Polym. J. 2015, 70, 400–411.
Kamal, M. R.; Khoshkava, V. Effect of cellulose nanocrystals (CNC) on rheological and mechanical properties and crystallization behavior of PLA/CNC nanocomposites. Carbohydr. Polym. 2015, 123, 105–114.
Bai, H.; Zhang, W.; Deng, H.; Zhang, Q.; Fu, Q. Control of crystal morphology in poly(L-lactide) by adding nucleating agent. Macromolecules 2011, 44, 1233–1237.
Bai, H.; Huang, C.; Xiu, H.; Zhang, Q.; Fu, Q. Enhancing mechanical performance of polylactide by tailoring crystal morphology and lamellae orientation with the aid of nucleating agent. Polymer 2014, 55, 6924–6934.
Shen, T.; Xu, Y.; Cai, X.; Ma, P.; Dong, W.; Chen, M. Enhanced crystallization kinetics of poly(lactide) with oxalamide compounds as nucleators: effect of spacer length between the oxalamide moieties. RSC Adv. 2016, 6, 48365–48374.
Bao, J.; Chang, X.; Xie, Q.; Yu, C.; Shan, G.; Bao, Y.; Pan, P. Preferential formation of β-form crystals and temperature-dependent polymorphic structure in supramolecular poly(L-lactic acid) bonded by multiple hydrogen bonds. Macromolecules 2017, 50, 8619–8630.
Kodal, M.; Sirin, H.; Ozkoc, G. Non-isothermal crystallization kinetics of PEG plasticized PLA/G-POSS nanocomposites. Polym. Compos. 2017, 38, 1378–1389.
Dave, V.; Srivastava, P.; Sharma, S.; Bajaj, J.; Tak, K. PEGylated PLA-Phospholipon 90G complex hybrid nanoparticles loaded with etoricoxib for effective treatment pain relief potential. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 640–652.
Xia, S.; Liu, X.; Wang, J.; Kan, Z.; Chen, H.; Fu, W.; Li, Z. Role of poly(ethylene glycol) grafted silica nanoparticle shape in toughened PLA-matrix nanocomposites. Compos. Part B 2019, 168, 398–405.
Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163.
Sijbrandi, N. J.; Kimenai, A. J.; Mes, E. P. C.; Broos, R.; Bar, G.; Rosenthal, M.; Odarchenko, Y. I.; Ivanov, D. A.; Feijen, J.; Dijkstra, P. J. Synthesis, morphology and properties of segmented poly(ether ester amide)s comprising uniform glycine or β-alanine extended bisoxalamide hard segments. Polymer 2012, 53, 4033–4044.
Harings, J. A.; van Asselen, O.; Graf, R.; Broos, R.; Rastogi, S. The role of superheated water on the crystallization of N,N′-1, 2-ethanediyl-bis(6-hydroxy-hexanamide): Implications on crystallography and phase transitions. Cryst. Growth Des. 2008, 8, 2469–2477.
Tsuji, H. In vitro hydrolysis of blends from enantiomeric poly(lactide)s. Part 4: well-homo-crystallized blend and nonblended films. Biomaterials 2003, 24, 537–547.
Takahiko, K.; Nelly, R.; Go, M.; Koji, N.; Toshiji, K.; Mitsuru, N.; Hirotaka, O.; Jumpei, K.; Arimitsu, U.; Nobutaka, H. Crystallization and melting behavior of poly(l-lactic acid). Macromolecules 2013, 40, 9463–9469.
Zhang, R. C.; Sun, D.; Lu, A.; Zhong, M.; Xiong, G.; Wan, Y. Equilibrium melting temperature of polymorphic poly(L-lactide) and its supercooling dependence on growth kinetics. Polymers 2017, 9, 625.
Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. II. Experimental evidence. J. Non-Cryst. Solids 1993, 162, 13–25.
Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Phys. Chem. 1941, 9, 177–184.
Tsuji, H.; Yamashita, Y. Highly accelerated stereocomplex crystallization by blending star-shaped 4-armed stereo diblock poly(lactide)s with poly(D-lactide) and poly(L-lactide) cores. Polymer 2014, 55, 6444–6450.
Shen, T.; Xu, Y.; Wang, L.; Dong, W.; Chen, M.; Ma, P. Highperformance poly(lactide) composites by construction of network-like shish-kebab crystals. RSC Adv. 2016, 6, 71046–71051.
Hoffman, J. D. Theoretical aspects of polymer crystallization with chain folds: bulk polymers. Polym. Eng. Sci. 1964, 4, 315–362.
Shen, T.; Ma, P.; Yu, Q.; Dong, W.; Chen, M. The effect of thermal history on the fast crystallization of poly(L-lactide) with soluble-type nucleators and shear flow. Polymers 2016, 8, 431.
Tsuji, H.; Miyase, T.; Tezuka, Y.; Saha, S. K. Physical properties, crystallization, and spherulite growth of linear and 3-arm poly(L-lactide)s. Biomacromolecules 2007, 100, 944–947.
Zhang, M.; Guo, B. H.; Xu, J. A review on polymer crystallization theories. Crystals 2017, 7, 4.
Tang, X.; Chen, W.; Li, L. The tough journey of polymer crystallization: battling with chain flexibility and connectivity. Macromolecules 2019, 52, 3575–3591.
Okada, K.; Watanabe, K.; Urushihara, T.; Toda, A.; Hikosaka, M. Role of epitaxy of nucleating agent (NA) in nucleation mechanism of polymers. Polymer 2007, 48, 401–408.
Legras, R.; Mercier, J.; Nield, E. Polymer crystallization by chemical nucleation. Nature 1983, 304, 432.
Xing, Q.; Li, R.; Dong, X.; Luo, F.; Kuang, X.; Wang, D.; Zhang, L. Enhanced crystallization rate of poly(L-lactide) mediated by a hydrazide compound: nucleating mechanism study. Macromol. Chem. Phys. 2015, 216, 1134–1145.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51873082), the MOE & SAFEA 111 Project (No. B13025), the Opening Project of Beijing Key Laboratory of Quality Evaluation Technology for Hygiene and Safety of Plastics (Beijing Technology and Business University) (No. QETHSP2019003), and the Postgraduate Research & Practice Innovation Program of Jiangnan University (No. JNKY19_020).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Information
10118_2020_2461_MOESM1_ESM.pdf
Enhancing the Crystallization Performance of Poly(L-lactide) by Intramolecular Hybridizing with Tunable Self-assembly-type Oxalamide Segments
Rights and permissions
About this article
Cite this article
Yu, MM., Yang, WJ., Niu, DY. et al. Enhancing the Crystallization Performance of Poly(L-lactide) by Intramolecular Hybridizing with Tunable Self-assembly-type Oxalamide Segments. Chin J Polym Sci 39, 122–132 (2021). https://doi.org/10.1007/s10118-020-2461-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-020-2461-3