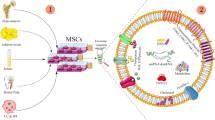
Abstract
Parkinson’s disease (PD) is a chronic, progressive, neurodegenerative disease. The predominant pathology of PD is the loss of dopaminergic cells in the substantia nigra. Cell transplantation is a strategy with significant potential for treating PD; mesenchymal stem cells (MSCs) are a tremendous therapeutic cell source because they are easily accessible. MSC-derived exosomes with potential protective action in lesioned sites serve as an essential promoter of neuroprotection, and neurodifferentiation, by modulating neural stem cells, neurons, glial cells, and axonal growth in vitro and in vivo environments. The biological properties of MSC-derived exosomes have been proposed as a beneficial tool in different pathological conditions, including PD. Therefore, in this review, we assort the current understanding of MSC-derived exosomes as a new possible therapeutic strategy for PD by providing an overview of the potential role of miRNAs as a component of exosomes in the cellular and molecular basis of PD.


Similar content being viewed by others
Data availability
This is a review article. There is no consent to publish applicable.
Abbreviations
- PD :
-
Parkinson’s disease
- MSCs :
-
mesenchymal stem cells
- miRNAs :
-
microRNAs
- MAO-B :
-
monoamine oxidase-B
- COMT :
-
catechol-o-methyltransferase
- EVs :
-
membrane-bound extracellular vesicles
- CCV :
-
clathrin-coated vesicles
- MVBs :
-
multi-vesicular bodies
- ILVs :
-
intraluminal vesicles
- TGN :
-
trans-Golgi Network
- ESCRT :
-
endosomal sorting complex required for transport
- SNAREs :
-
soluble N-ethylmaleimide sensitive factor attachment protein receptors
- LncRNA :
-
long non-coding RNA
- CNS :
-
central nervous system
- CNS :
-
central nervous system
- NPCs :
-
neural progenitor cells
- siRNAs :
-
short interfering RNAs
- BBB :
-
blood-brain barrier
- BMECs :
-
brain microvascular endothelial cells
- GluR :
-
glutamate receptors R
- L1CAM :
-
L1 cell adhesion molecule
- LBs :
-
Lewy bodies
- Aβ :
-
amyloid beta
- MHC-1 :
-
major histocompatibility complex I
- SNpc :
-
substantia nigra pars compacta
- PEDF :
-
pigment epithelium-derived factor
- Cys-C :
-
cystatin-C
- BDNF :
-
brain-derived neurotrophic factor
- IGF-1 :
-
insulin-like growth factor 1
- VEGF :
-
vascular endothelial growth factor
- MMPs :
-
matrix metalloproteinases
- UTR :
-
untranslated region
- ORF :
-
open reading frame
- TLR :
-
toll-like receptor
- CSF :
-
cerebrospinal fluid
- ROCK1 :
-
Rho-associated kinase 1
- SNCA :
-
synuclein alpha
- MPP + :
-
1-methyl-4-phenylpyridinium
- NK :
-
neural killer
- IFN-γ :
-
interferon gamma
- IL :
-
interleukin
- IRS-1 :
-
insulin receptor substrate 1
- MDS-UPDRS :
-
Movement Disorders Society Unified Parkinson’s Disease Rating Scale
- AMSCs :
-
adipose mesenchymal stem cells
- MALAT1 :
-
metastasis-associated lung adenocarcinoma transcript 1
- UMSCs :
-
umbilical cord mesenchymal stem cells
- HDAC :
-
histone deacetylase
References
Mizuno Y et al (1995) Role of mitochondria in the etiology and pathogenesis of Parkinson’s disease. Biochim Biophys Acta (BBA)- Mol Basis Dis 1271(1):265–274
Rizek P, Kumar N, Jog MS (2016) An update on the diagnosis and treatment of Parkinson disease. CMAJ 188(16):1157–1165
Chen H, Ritz B (2018) The search for environmental causes of Parkinson’s disease: moving forward. J Parkinsons Dis 8(s1):S9–S17
Fox SH et al (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33(8):1248–1266
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. JAMA 323(6):548–560
Yu H et al (2020) Potential roles of exosomes in Parkinson’s disease: from pathogenesis, diagnosis, and treatment to prognosis. Front Cell Dev Biol 8:86
Barbuti PA et al (2021) Recent advances in the development of stem-cell-derived dopaminergic neuronal transplant therapies for Parkinson’s disease. Mov Disord 36(8):1772–1780
Yuan O et al (2019) Exosomes derived from human primed mesenchymal stem cells induce mitosis and potentiate growth factor secretion. Stem Cells Dev 28(6):398–409
Fayazi N et al (2021) Stem cell-derived exosomes: a new strategy of neurodegenerative disease treatment. Mol Neurobiol 58(7):3494–3514
Goetzl L et al (2018) Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann Clin Transl Neurol 5(1):4–10
Gui Y et al (2015) Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 6(35):37043
Jin Q et al (2021) Extracellular vesicles: novel roles in neurological disorders. Stem Cells Int 2021:1–16
Koong AC, Chen EY, Giaccia AJ (1994) Hypoxia causes the activation of nuclear factor κB through the phosphorylation of IκBα on tyrosine residues. Can Res 54(6):1425–1430
Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Urbanelli L et al (2013) Signaling pathways in exosomes biogenesis, secretion and fate. Genes 4(2):152–170
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478):eaau6977
Ostrowski M et al (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12(1):19–30
Sluijter JP (2013) MicroRNAs in cardiovascular regenerative medicine: directing tissue repair and cellular differentiation. Int Schl Res Notices 2013
Ma Y et al (2019) Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun Signal 17(1):1–10
Gao G et al (2019) Glutaminase C regulates microglial activation and pro-inflammatory exosome release: relevance to the pathogenesis of Alzheimer’s disease. Front Cell Neurosci 13:264
Emmanouilidi A et al (2019) Oncogenic and non-malignant pancreatic exosome cargo reveal distinct expression of oncogenic and prognostic factors involved in tumor invasion and metastasis. Proteomics 19(8):1800158
Mathivanan S et al (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73(10):1907–1920
Fu S et al (2020) Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact 20:100261
Gao G et al (2020) Glutaminase 1 regulates neuroinflammation after cerebral ischemia through enhancing microglial activation and pro-inflammatory exosome release. Front Immunol 11:161
Ma Y et al (2019) Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal–regulated kinase pathways. Neurobiol Dis 124:322–334
Ludwig N et al (2019) Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci 20(19):4684
Gilligan KE et al (2017) Engineering exosomes for cancer therapy. Int J Mol Sci 18(6):1122
Jakubec M et al (2020) Plasma-derived exosome-like vesicles are enriched in lyso-phospholipids and pass the blood-brain barrier. PLoS ONE 15(9):e0232442
Zhao Y et al (2020) Paracrine interactions involved in human induced pluripotent stem cells differentiation into chondrocytes. Curr Stem Cell Res Ther 15(3):233–242
Zolnik BS et al (2010) Minireview: nanoparticles and the immune system. Endocrinology 151(2):458–465
Dézsi L et al (2014) Features of complement activation-related pseudoallergy to liposomes with different surface charge and PEGylation: comparison of the porcine and rat responses. J Control Release 195:2–10
Gamucci O et al (2014) Biomedical nanoparticles: overview of their surface immune-compatibility. Coatings 4(1):139–159
D’Anca M et al (2019) Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front Aging Neurosci 11:232
He M et al (2021) Diagnostic and therapeutic potential of exosomal microRNAs for neurodegenerative diseases. Neural Plast 2021:1–13
Chen CC et al (2016) Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529
Liu R et al (2021) Mesenchymal stem cell-derived exosomes for treatment of ischemic stroke. Curr Stem Cell Res Ther 12:561
Wang X et al (2020) The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid Med Cell Longev 2020:17
Ebrahimi M et al (2020) Differentiation of human induced pluripotent stem cells into erythroid cells. Stem Cell Res Ther 11(1):1–13
Yuan L, Li J-Y (2019) Exosomes in Parkinson’s disease: current perspectives and future challenges. ACS Chem Neurosci 10(2):964–972
Pinnell JR, Cui M, Tieu K (2021) Exosomes in Parkinson disease. J Neurochem 157(3):413–428
Liu L et al (2020) Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing α-synuclein and immune activation of Parkinson’s disease. Sci Adv 6(50):eaba3967
D’Anca M et al (2019) Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front Aging Neurosci 11:232
Danzer KM et al (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7(1):1–18
Chivet M et al (2014) Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3(1):24722
Emmanouilidou E et al (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30(20):6838–6851
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Harischandra DS et al (2018) Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to α-synuclein misfolding in metal neurotoxicity. Neurotoxicology 64:267–277
Vallelunga A et al (2014) Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and Multiple System Atrophy. Front Cell Neurosci 8:156
Dos Santos MCT et al (2018) miRNA-based signatures in cerebrospinal fluid as potential diagnostic tools for early-stage Parkinson’s disease. Oncotarget 9(25):17455
Li D et al (2018) Effect of regulatory network of exosomes and microRNAs on neurodegenerative diseases. Chin Med J. 131(18):2216–2225
Tan L et al (2015) Causes and consequences of microRNA dysregulation in neurodegenerative diseases. Mol Neurobiol 51(3):1249–1262
Batistela MS et al (2017) An overview of circulating cell-free microRNAs as putative biomarkers in Alzheimer’s and Parkinson’s Diseases. Int J Neurosci 127(6):547–558
Xin H et al (2017) MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48(3):747–753
Baglio SR et al (2015) Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6(1):1–20
Vilaça-Faria H et al (2019) Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease? Cells. 8(2):118
Xin H et al (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cellscontributes to neurite outgrowth. Stem Cells 30(7):1556–1564
Martins M et al (2011) Convergence of miRNA expression profiling, α-synuclein interacton and GWAS in Parkinson’s disease. PloS One 6(10):e25443
Alvarez-Erviti L et al (2013) Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson’s disease. Cell Death Dis 4(3):e545–e545
Burgos K et al (2014) Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PloS One 9(5):e94839
Doxakis E (2010) Post-transcriptional regulation of α-synuclein expression by mir-7 and mir-153. J Biol Chem 285(17):12726–12734
Kabaria S et al (2015) Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett 589(3):319–325
Ponomarev ED, Veremeyko T, Weiner HL (2013) MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61(1):91–103
Vizoso FJ et al (2017) Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 18(9):1852
Kojima R et al (2018) Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun 9(1):1–10
Cooper JM et al (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29(12):1476–1485
Shakespear N et al (2020) Astrocyte-derived exosomal microRNA miR-200a-3p prevents MPP+-induced apoptotic cell death through down-regulation of MKK4. Neurochem Res 45(5):1020–1033
McMillan KJ et al (2017) Loss of microRNA-7 regulation leads to α-synuclein accumulation and dopaminergic neuronal loss in vivo. Mol Ther 25(10):2404–2414
Baba Y et al (2005) Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord 11(8):493–498
Glennie S et al (2005) Mesenchymal stem cells induce division arrest anergy of activated T cells. Biol Blood Marrow Transplant 11(2):1
Chen J et al (2019) Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin α5 in hepatocellular carcinoma. Cell Death Dis 10(6):1–12
Hoogduijn MJ, Lombardo E (2019) Mesenchymal stromal cells anno 2019: dawn of the therapeutic era? Concise review. Stem Cells Transl Med 8(11):1126–1134
Qiu X et al (2020) Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif 53(8):e12830
Molchanova E et al (2011) The sensitivity of mesenchymal stromal cell subpopulations with different times of adhesion property manifestation and derived from hemopoietic organs to growth factors EGF, bFGF, and PDGF. Izv Akad Nauk Ser Biol 2:133–144
Cho D-I et al (2014) Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 46(1):e70–e70
Vasandan AB et al (2016) Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep 6(1):1–17
Phinney DG, Pittenger MF (2017) MSC-derived exosomes for cell-free therapy stem cells. Stem Cells 35:851–858
Marote A et al (2016) MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol 7(2016):231
Teixeira FG et al (2013) Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci 70(20):3871–3882
Salgado AJ et al (2015) Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci 9:249
Takayama Y et al (2021) Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J Control Release 329:1090–1101
Sordi V (2009) Mesenchymal stem cell homing capacity. Transplantation 87(9S):S42–S45
Meng Q-S et al (2020) Senescent mesenchymal stem/stromal cells and restoring their cellular functions. World J Stem Cells 12(9):966
Joyce N et al (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5(6):933–946
Dominici M et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Li M, Ikehara S (2013) Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int 2013:132642
Bian S et al (2014) Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med 92(4):387–397
Arslan F et al (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10(3):301–312
Zhang B et al (2014) Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 23(11):1233–1244
Tan CY et al (2014) Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 5(3):1–14
Teixeira FG et al (2016) Modulation of the mesenchymal stem cell secretome using computer-controlled bioreactors: impact on neuronal cell proliferation, survival and differentiation. Sci Rep 6(1):1–14
Assunção-Silva RC et al (2018) Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie 155:83–91
Pires AO et al (2016) Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev 25(14):1073–1083
Jarmalavičiūtė A et al (2015) Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxydopamine–induced apoptosis. Cytotherapy 17(7):932–939
Fan X-L et al (2020) Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci 77(14):2771–2794
Harrell CR et al (2019) Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8(5):467
Gong M et al (2017) Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8(28):45200
Sonntag K-C (2010) MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res 1338:48–57
Ha D, Yang N, Nadithe V (2016) (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6(4):287–296
Alvarez-Erviti L et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotech 29(4):341–345
Kim W et al (2014) miR-126 contributes to Parkinson disease by dysregulating IGF-1/PI3K signaling: miRNAs and IGF-1 signaling in Parkinson disease. Neurobiol Aging 35(7):1712
Haney MJ et al (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30
Lang FM et al (2018) Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-Oncol 20(3):380–390
Lee S-T et al (2017) Exosome-based delivery of miR-124 in a Huntington’s disease model. J Mov Dis 10(1):45
Athauda D et al (2019) Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol 76(4):420–429
Mathieu M et al (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21(1):9–17
Witwer KW et al (2019) Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 8(1):1609206
Acknowledgments
The authors would like to thank Dr. Seyed Esmaeil Khoshnam for suggesting the subject of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, drafting the article or revising it critically for important intellectual content, and approval of the final version.
1. MA contributed to designing and preparing the manuscript.
2. MJ contributed to preparation of manuscript and editing it.
3. NY contributed to preparing figures and table and revising the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is a review article. There is no ethical approval applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abrishamdar, M., Jalali, M.S. & Yazdanfar, N. The role of exosomes in pathogenesis and the therapeutic efficacy of mesenchymal stem cell-derived exosomes against Parkinson’s disease. Neurol Sci 44, 2277–2289 (2023). https://doi.org/10.1007/s10072-023-06706-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06706-y